Clinical Studies of Lung Damage
A biomarker is a measurable substance, usually in blood, whose presence indicates a disease, infection, or environmental exposure to a harmful agent. While Echelon ELISA kits are sold as Research Use Only (RUO), scientists and researchers from around the world frequently use these products for interesting clinical studies. Two recent studies using a Sphingosine 1-phosphate (S1P) ELISA (K-1900) from Echelon Biosciences determined that S1P is a possible biomarker for dangerous inflammatory lung disorders.
First, researchers in Nanjing China evaluated 121 patients in the intensive care unit that had acute respiratory distress syndrome (ARDS) and compared them with 100 healthy controls for blood markers of inflammation including S1P (Zhao, 2020). A second study was performed by clinical researchers in Milan Italy who evaluated 111 patients with COVID-19 disease compared to 47 healthy individuals. In both studies, serum levels of S1P were significantly decreased in those people suffering from respiratory distress (Marfia, 2020).
S1P and Acute Respiratory Distress Syndrome
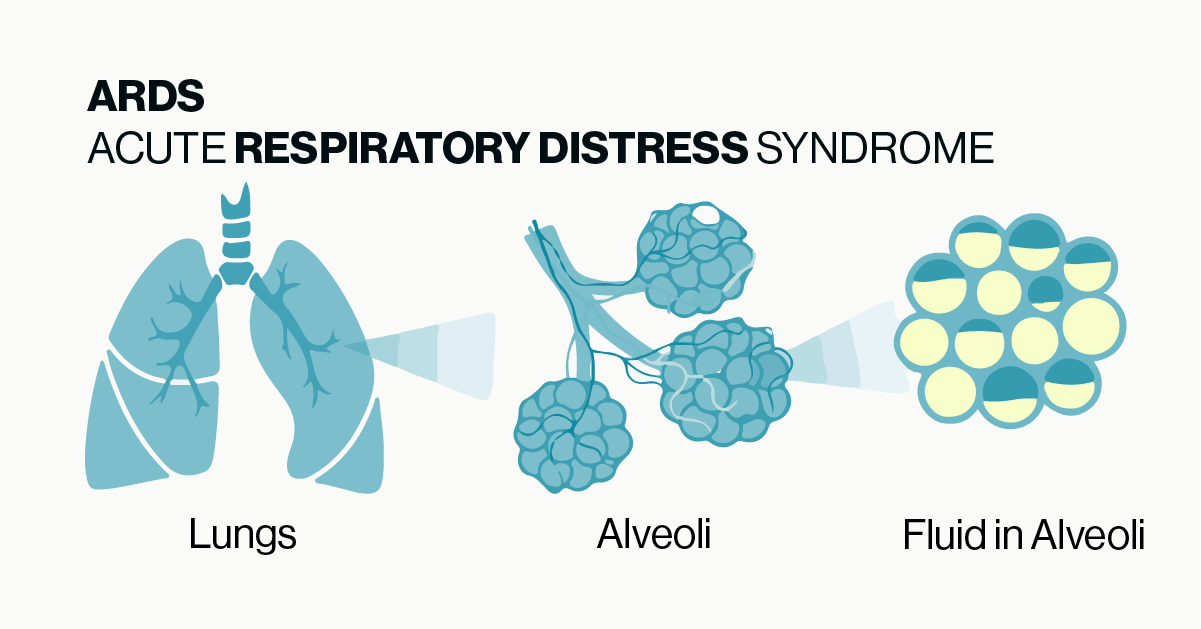
ARDS is a life-threatening lung injury which is caused by a number of insults or microbes that induce harmful inflammation including excessive immune cell activation and cytokine production. The pathology of ARDS is fairly straightforward and is illustrated in Figure 1. Under normal conditions gas exchange in the lungs occurs between the alveoli and blood vessels within the lungs. Damage or inflammation to the blood vessels can cause fluid to leak out and build up within the alveoli which then blocks the exchange of oxygen and carbon dioxide. While the symptoms and tissue pathology of ARDS are fairly well defined, the underlying molecular mechanisms have not been fully characterized.
Jiangnan Zhao and colleagues designed a multi-center prospective study to determine that serum S1P levels were significantly lower in ARDS patients than in healthy controls and that reduced S1P was associated with more organ dysfunction and higher mortality. These patients were enrolled and all study procedures completed before the current COVID-19 outbreak caused by the respiratory virus SARS-CoV-2. Indeed, bacterial infection in the blood (sepsis) was associated with lower S1P levels and more severe ARDS compared to other lung-associated conditions, aspiration and pneumonia, highlighting the fact that our lungs can be damaged from the outside via the airway and also from the inside via the blood. So, what about severe acute respiratory syndrome (SARS) and the current pandemic?
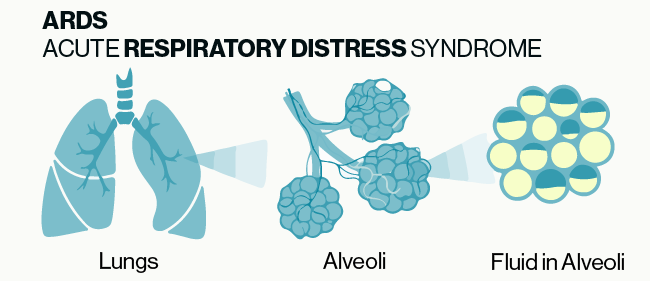
Figure 1: Simple illustration of the basic pathology of ARDS. Damage or inflammation to the vessels and tissue surrounding alveoli can cause build up of fluid in the alveoli which prevents the normal exchange of gases in the lungs.
S1P and Severe Acute Respiratory Syndrome
COVID-19 is the disease caused by the novel coronavirus, SARS-CoV-2. The infectious agent is a respiratory virus with some patients developing life-threatening SARS. When the virus enters the body, it attaches to epithelial cells in the upper airway, which triggers an immune response that causes inflammation. This inflammatory response is what leads to the commonly reported symptoms of cough, sore throat, and fever. In cases of greater exposure or viral load, the virus will move deeper into the lungs and can attach in the alveoli. This scenario is when COVID-19 can lead to ARDS, typically presenting about a week after the onset of initial symptoms. Pre-exisiting conditions and underlying factors such as advanced age, diabetes, and high blood pressure increase the likelihood of the development of ARDS in people with COVID-19.
Giovanni Marfia, Stefania Navone, and colleagues found that serum S1P levels are significantly lower in COVID-19 patients presenting at the hospital compared to healthy volunteers. S1P as a biomarker predicted both ICU admission and mortality. The authors discuss several possible mechanisms for why S1P levels are decreased during SARS based on the cells and transporters in blood known to produce, degrade, and bind to S1P. They further speculate that depressed S1P in serum could cause lymphopenia—a condition where important virus-fighting cells are trapped in lymph nodes and other tissues, and blocked from relocating to the lungs and other organs to mount effective immune response against the CARS-CoV-2 virus and/or contributes to the “cytokine storm” observed in serious cases of COVID-19. Whether these mechanisms are accurate or not will require additional clinical investigation. At Echelon Biosciences, we pledge to continue making quality research reagents, tools, and assays to enable scientists working to understand disease and improve human health.
References
1. Zhao J, Tan Y, Wang L, Su X, Shi Y. Serum sphingosine-1-phosphate levels and Sphingosine-1-Phosphate gene polymorphisms in acute respiratory distress syndrome: a multicenter prospective study. Journal of Translational Medicine 2020; 18(1):156.
2. Marfia G, Navone S, Guarnaccia L, Campanella R, Mondoni M, Locatelli M, Barassi A, Fontana L, Palumbo F, Garzia E, Ciniglio Appiani G, Chiumello D, Miozzo M, Centanni S, Riboni L. Decreased serum level of sphingosine-1-phosphate: a novel predictor of clinical severity in COVID-19. EMBO Molecular Medicine; n/a(n/a):e13424.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences