Recombinant C-terminal His-tagged, MEP Synthase (DXR, 2-C-methyl-D-erythritrol-4-phosphate synthase)
Storage: Store product > -20 ºC. Enzyme is stable for at least 6 months at -20 ºC as undiluted stock
Molecular Weight: 45.0 kDa
Specific Activity: > 2 Units / mg (1 Unit of DXR activity is defined as the oxidation of 1 µmol of β-NADPH / minute using 120 µM DXP as substrate with 10 nM DXR for two minutes at 37 °C.) Request Certificate of Analysis (COA) for lot specific information.
Product Description:
The recombinant C-terminal His-tagged fusion protein is purified from E.coli using Ni-NTA column chromatography.
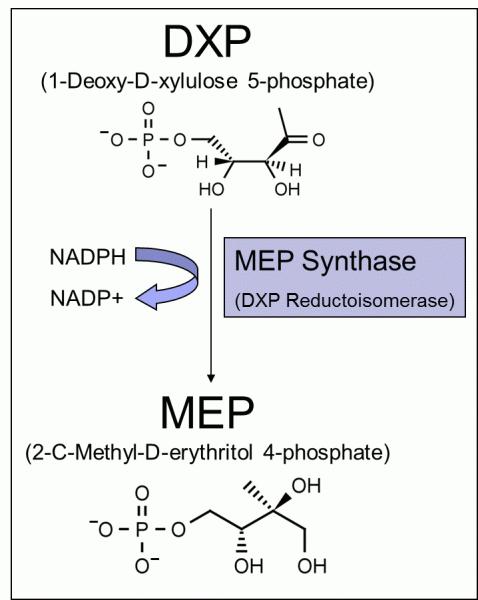
In the first pathway-specific reaction of the methylerythritol phosphate (MEP) pathway for isoprenoid biosynthesis, MEP Synthase (DXR) catalyzes the rearrangement of 1-deoxy-D-xylulose-5-phosphate (DXP) to generate 2-C-methyl-D-erythritrol-4-phosphate (MEP) in the presence of B-nicotinamide adenine dinucleotide phosphate (NADPH) and a divalent cation [1].
Fosmidomycin inhibits MEP synthase in many organisms and has validated the MEP pathway as a potential antibiotic pathway.The MEP pathway is used by most bacteria, including all Gram-negative bacteria, for isoprenoid biosynthesis. Isoprenoids comprise one of the most diverse classes of compounds found in nature. With over 50,000 different isoprenoids identified to date, they exhibit a broad range of structural complexity and are involved in a variety of biological functions [2]. Electron transport (quinones), stabilization of cell membranes (hopanoids and sterols), cell wall biosynthesis (dolichols), signal transduction (prenylated proteins), photosynthesis (chlorophylls) and modification of tRNAs are among the processes that involve isoprenoids [3]. Isopentenyl diphosphate (IPP) and dimethylallyl disphosphate (DMAPP) are the precursors for all isoprenoid compounds and two unrelated essential pathways exist in nature for their biosynthesis. These two precursors are produced by either the mevalonate (MVA) or MEP pathway. The MVA pathway in found primarily in eukaryotes, including humans, plant cytosol, Archaea, and some Gram-positive bacteria, while the MEP pathway is utilized by most bacteria and plant chloroplasts. Due to this natural distribution, the MEP pathway represents a promising target for development of novel antibacterial agents and herbicides [4].
References:
1) Bochar, D.A.; Freisen, J.A.; Stauffacher, C.V. and Rodwell, V.W.(1999) in Comprehensive Natural Products Chemistry, (Cane, D. Ed.) Pergamon Press, Oxford, pp. 15-44.
2) Sacchettini, J.C. and Poulter, C.C. (1997) Science, 277(5333), 1788-9.
3) Testa, C.A.; Brown, M.J. (2003) Current Pharmceutical Biotechnology, 4, 248-259.