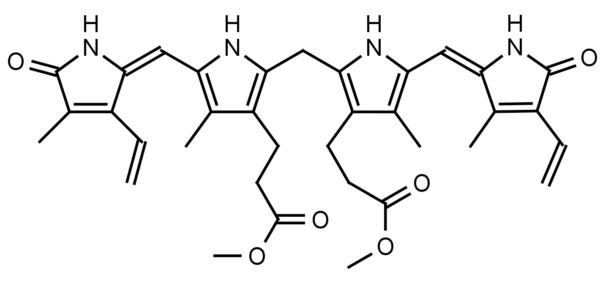
Bilirubin dimethyl ester is a natural derivative of bilirubin and is found in normal sera representing an average of 1.75% total sera bilirubin. Bilirubin is a water insoluble tetrapyrrole produced from the reduction of biliverdin in a reaction catalyzed by the enzyme bilirverdin reductase. Water insoluble bilirubin (also called indirect bilirubin) in vivo undergoes glucuronidation in the liver (addition of one or two glucuronic acids through a glycosidic bond) to form the water soluble bilirubin mono or diglucuronide (also called bilirubin conjugate or direct bilirubin). Bilirubin conjugate is excreted from the liver in bile or is converted to urobilinogen and excreted in the urine as urobilin or in the feces as stercobilin. Bilirubin dimethyl ester has been found to be converted to bilirubin conjugate via esterase and glucuronidase activity in vivo.
References
1) Muraca, M. and N. Blanckaert (1983). “Liquid-chromatographic assay and identification of mono- and diester conjugates of bilirubin in normal serum.” Clinical Chemistry 29(10): 1767-71.
2) Burchell, B. and G. B. Odell (1981). “A rat liver microsomal carboxyesterase and a bilirubin UDP-glucuronyl transferase are responsible for the formation of bilirubin glucuronides from bilirubin dimethyl ester.” FEBS Letters 135(2): 304.
3) Spasojevic, I., I. Batinic-Haberle, et al. (2001). “Manganese(III) Biliverdin IX Dimethyl Ester: A Powerful Catalytic Scavenger of Superoxide Employing the Mn(III)/Mn(IV) Redox Couple.” Inorganic Chemistry 40(4): 726.
4) MacLean, P. D., E. C. Drake, et al. (2007). “Bilirubin as an antioxidant in micelles and lipid bilayers: Its contribution to the total antioxidant capacity of human blood plasma.” Free Radical Biology and Medicine 43(4): 600.