Acidic pH is required in some intracellular organelles, but in the extracellular environment it can be highly damaging to cells. Extracellular acidity, known as acidosis, is a common pathological feature of ischemia and cancer and is known to activate a class of chloride channels that can trigger induced cell death. These proton-activated chloride (PAC) channels have been shown to have a variety of resting, active, and inactive states over a range of pH 4.0-6.0, however the exact mechanisms governing these states has not been fully described. Previous work has suggested that human PACs (hPAC) are at least partially regulated by the phosphoinositide PI(4,5)P2, but it is unclear whether this is specific for PIP2 or generalizable to other phosphoinositides.
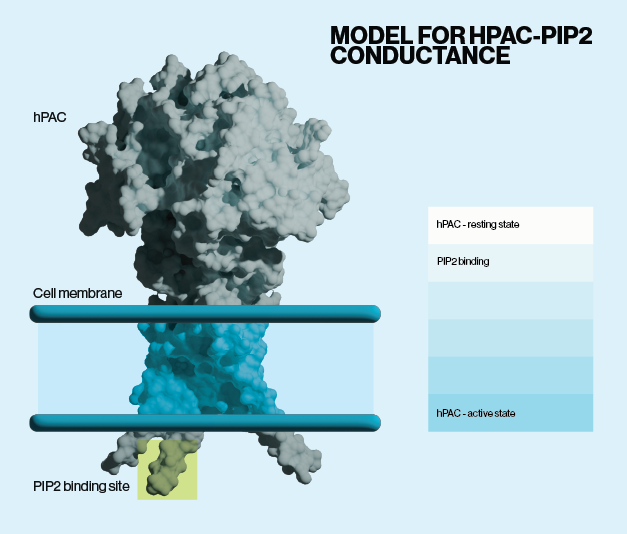
A recent study by Ko et al. explores the critical role of PI(4,5)P2 (Echelon P-4508) in the activation of PAC channels. They found that PI(4,5)P2, located specifically on the inner leaflet of the plasma membrane, is necessary for the activation of PAC channels. Depleting PI(4,5)P2 inhibited PAC activity, while applying the phospholipid to the cytoplasmic side of membranes increased activity. The authors used molecular docking simulations and alanine neutralization of basic residues of a specific transmembrane region to identify a putative binding site. This interaction is crucial for the channel’s function, specifically in opening the channel and stabilizing the active state of the PAC under conditions of extracellular acidification.
Given the well known signaling pathways that synthesize and degrade PI(4,5)P2, the authors then investigated whether activation of G-protein coupled receptors (GPCR) that increase hydrolysis of PI(4,5)P2 would decrease PAC activity. Stimulation of a class of GPCRs called muscarinic receptors led to a decrease in PI(4,5)P2 at the plasma membrane and a subsequent loss of PAC activity, even at low pH. Taken together, their findings provide new mechanistic insights to PAC activation and suggest a route by which acidosis may be modulated during acute and chronic pathological events.
Read the full article here:
Nature Communications (2024)
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences