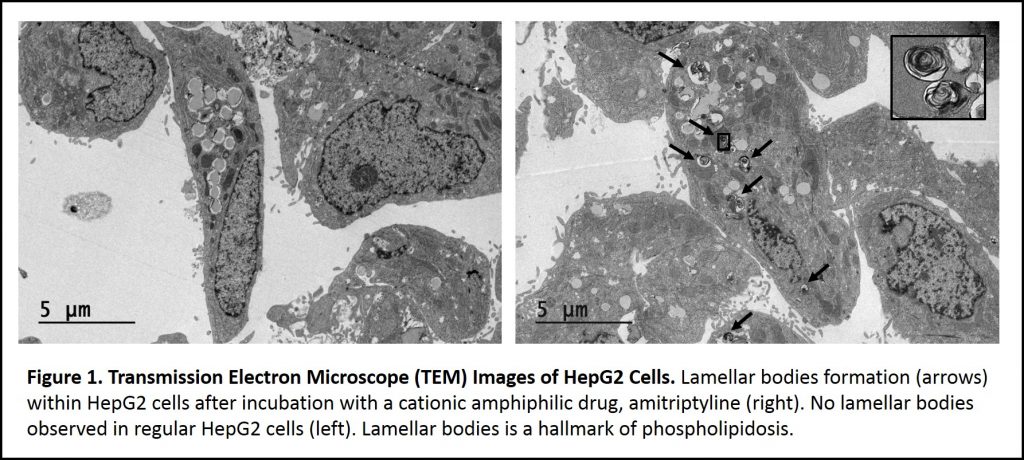
Phospholipidosis (PL) is lysosomal storage disorder characterized by the excessive accumulation of intracellular phospholipids (Figure 1). The mechanism of phospholipidosis is still unclear today, but it is known that phospholipidosis is commonly caused by the intake of a special group of drugs containing a hydrophobic ring/chain and a hydrophilic amine group termed, cationic amphiphilic drugs (CADs). This type of PL is referred to as drug-induced phospholipidosis (DIPL). It is generally accepted that the interaction between the hydrophobic and hydrophilic regions of both phospholipids and cationic amphiphilic drugs causes the phospholipid accumulation1.
Why does phospholipidosis matter?
The seriousness of DIPL can be explained in the case study reported by Gonzalez-Rothi et al. in the 1990s, in which a 62-year old woman was admitted to a Florida hospital with difficulty breathing. Her condition was first misdiagnosed as bronchitis. A year later, with multiple admissions to the hospital, it was identified that her breathing condition was actually the result of phospholipidosis. In her case, it was triggered by the antidepressant fluoxetine hydrochloride (Prozac™) which she had been taking on-and-off throughout the year2. DIPL is a reversible process; however, if not caught in time, the results can be deadly3.
How to know if a drug induces phospholipidosis
Since drug-induced phospholipidosis is often caused by cationic amphiphilic drugs, pharmaceutical companies often design drugs without the hydrophobic ring/chain and a hydrophilic amine group to avoid the potential phospholipidosis side effect. However, since the phospholipidosis mechanism is still unclear, a time consuming, high-cost cell-based transmission electron microscope (TEM) screen (Figure 1) is needed to rule out this potential side effect. For monitoring phospholipidosis events in patients, this cell-based transmission electron microscope method requires the correct type of specimen for the diagnosis, such as alveolar macrophages which are difficult to collect. Lysobisphosphatidic acid (LBPA), also known as bis(monoacylglycerol) phosphate (BMP), is suggested as a biomarker to monitor phospholipidosis4. However, this method requires sophisticated liquid chromatography with tandem mass spectrometry (LC-MS/MS) that is pre-set up for lysobisphosphatidic acid detection5.
Is there a simple method to test a compound for phospholipidosis?
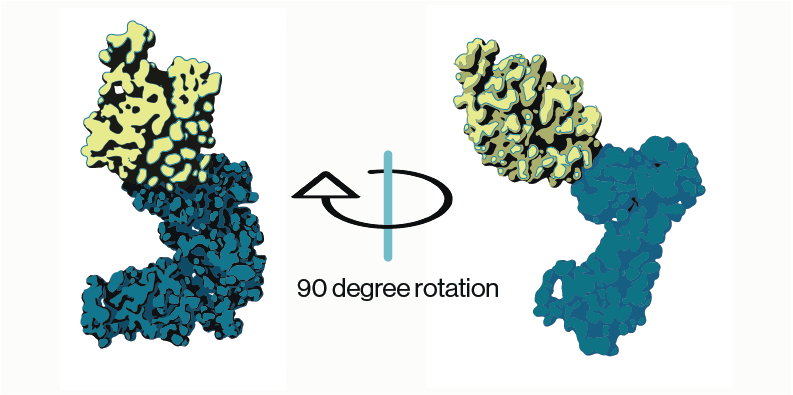
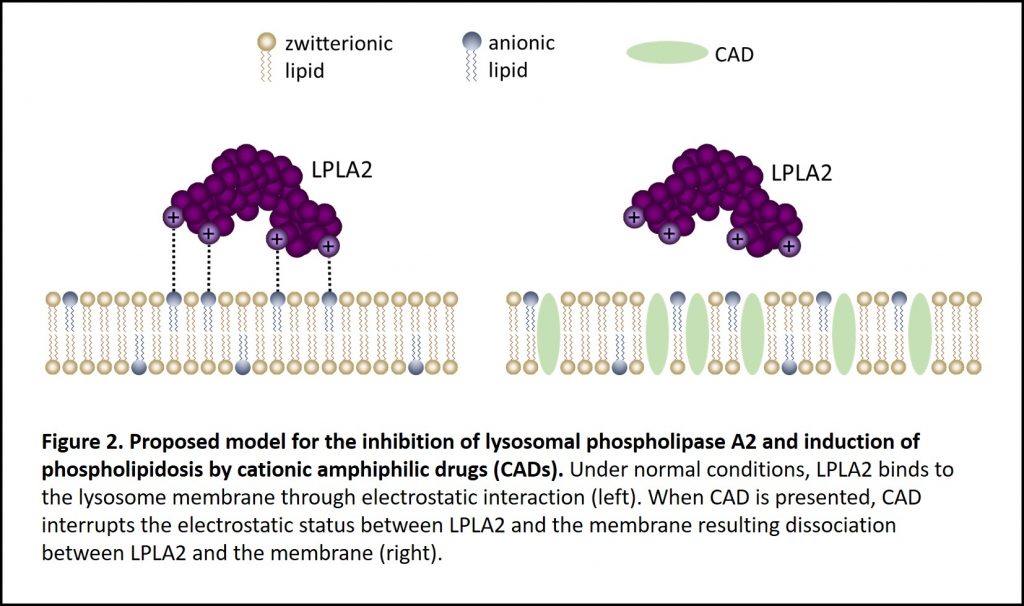
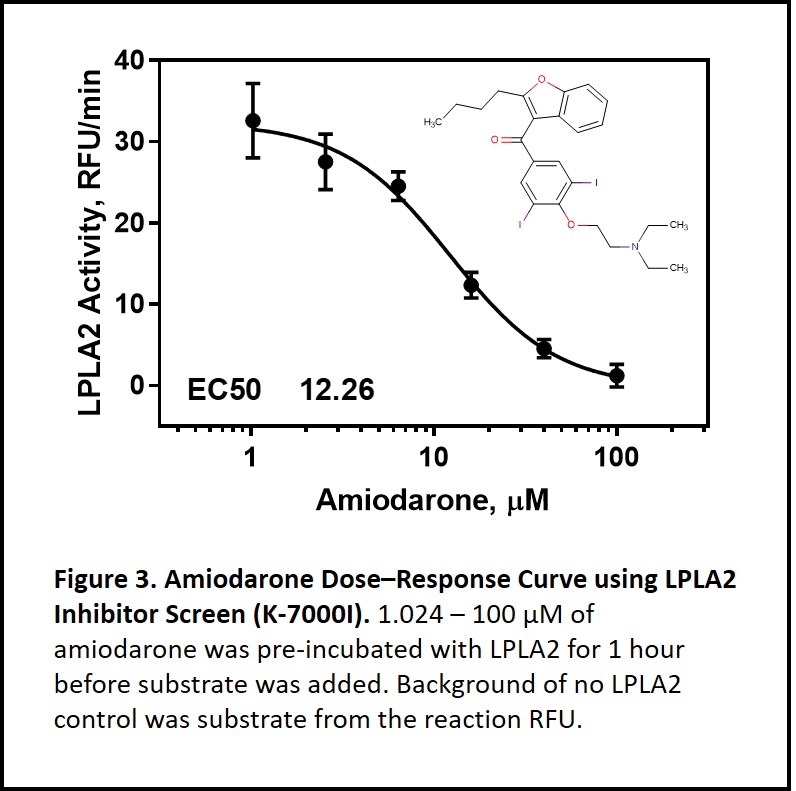
Yes. Lysosomal phospholipase A2 (LPLA2), also known as phospholipase A2 Group XV, is the only enzyme so far to be mechanistically linked to phospholipidosis. LPLA2 knockout mice present similar features seen in drug-induced phospholipidosis including foam cells with lamellar inclusion bodies and surfactant lipid accumulation5. Based on the LPLA2 structure, it is proposed that cationic amphiphilic drugs insert into the inner membrane of lysosome. These insertion events interrupt LPLA2 electrostatic charge interactions with the inner membrane resulting in phospholipids accumulation i.e. drug-induced phospholipidosis (Figure 2)6-7. Using Echelon’s LPLA2 Inhibitor Screen (catalog no. K-7000I), we were able to demonstrate amiodarone inhibition of LPLA2 in a dose-dependent manner (Figure 3). Amiodarone is a widely used antiarrhythmic drug known to induce phospholipidosis. This simple enzymatic screen can be performed without sophisticated instruments and is scalable for high throughput drug screening. The full study of identifying drugs’ phospholipidosis property using the LPLA2 Inhibitor Screen can be found here.
 Check out Echelon's full Lysosomal Phospholipase A2 product line here
Check out Echelon's full Lysosomal Phospholipase A2 product line here
References
- Kodavanti, U. P.; Mehendale, H. M., Cationic amphiphilic drugs and phospholipid storage disorder. Pharmacol Rev 1990, 42 (4), 327-54.
- Gonzalez-Rothi, R. J.; Zander, D. S.; Ros, P. R., Fluoxetine hydrochloride (Prozac)-induced pulmonary disease. Chest 1995, 107 (6), 1763-5.
- Richer, M.; Robert, S., Fatal hepatotoxicity following oral administration of amiodarone. Ann Pharmacother 1995, 29 (6), 582-6.
- Liu, N.; Tengstrand, E. A.; Chourb, L.; Hsieh, F. Y., Di-22:6-bis(monoacylglycerol)phosphate: A clinical biomarker of drug-induced phospholipidosis for drug development and safety assessment. Toxicol Appl Pharmacol 2014, 279 (3), 467-76.
- Hiraoka, M.; Abe, A.; Lu, Y.; Yang, K.; Han, X.; Gross, R. W.; Shayman, J. A., Lysosomal phospholipase A2 and phospholipidosis. Mol Cell Biol 2006, 26 (16), 6139-48.
- Shayman, J. A.; Abe, A., Drug induced phospholipidosis: an acquired lysosomal storage disorder. Biochim Biophys Acta 2013, 1831 (3), 602-11.
- Shayman, J. A.; Tesmer, J. J. G., Lysosomal phospholipase A2. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864 (6), 932-940.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences