Since when is anything smaller than a micron called, “large”? When a single cell needs to ingest a large particle, it executes a process called phagocytosis. From the Greek “to eat” and “cell”, phagocytosis was first noted by the Canadian physician William Osler in 1876 and was studied and named a few years later by the Russian zoologist Élie Metchnikoff. When cells do eat other cells it’s not brutal cannibalism. Cells actively die of natural causes (apoptosis) and their building blocks need to be recycled. Organisms employ a special type of phagocytosis called efferocytosis to ingest and recycle cellular debris. However, the molecular details of these processes have yet to be fully elucidated.
Phosphatidylserine as an ‘eat me’ signal
A common assumption is that cell membrane components were intimately involved in phagocytosis and efferocytosis. However, it is only more recently that specific molecules and their roles in phagocytosis and efferocytosis have been identified. Phosphatidylserine (PS) is a well-recognized ‘eat me’ signal exposed on the outer surface of dying cells. A phagocytic cell recognizes PS by several protein receptors on its surface. This binding initiates a cascade of signaling events inside the phagocyte cell that results in engulfment and internalization of the apoptotic body. Neighboring cells then use the ingested material after it is further broken down and recycled. Despite the origin of the name, phagocytosis, non-cellular particles like microbeads can also be ingested. This provides an avenue to develop new tools to help researchers better understand the details of apoptosis, phagocytosis, and efferocytosis.
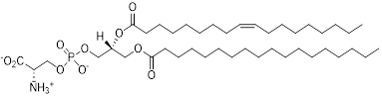
Figure 1: Representative structure of PS with 18:0 and 18:1 acyl chains.
Lipid Microparticles
Echelon has recently introduced Lipid Microparticles to our Lipid-Protein Interaction Tools product line. Standard Lipid Microparticles are approximately 3 µm in size with attached phospholipids and the option of a fluorophore. This size and the added fluorescence accommodate analysis with typical flow cytometry and microscopy methods. We currently provide Phosphatidylserine (PS) and Phosphatidylcholine (PC) coated microparticles with headgroups exposed on the outer surface for biological interactions. As PS is typically only found on the extracellular face of the cell during apoptosis, the PS Microparticles mimic this apoptotic signal. Conversely, the PC Microparticles should be ingested at a much lower frequency.
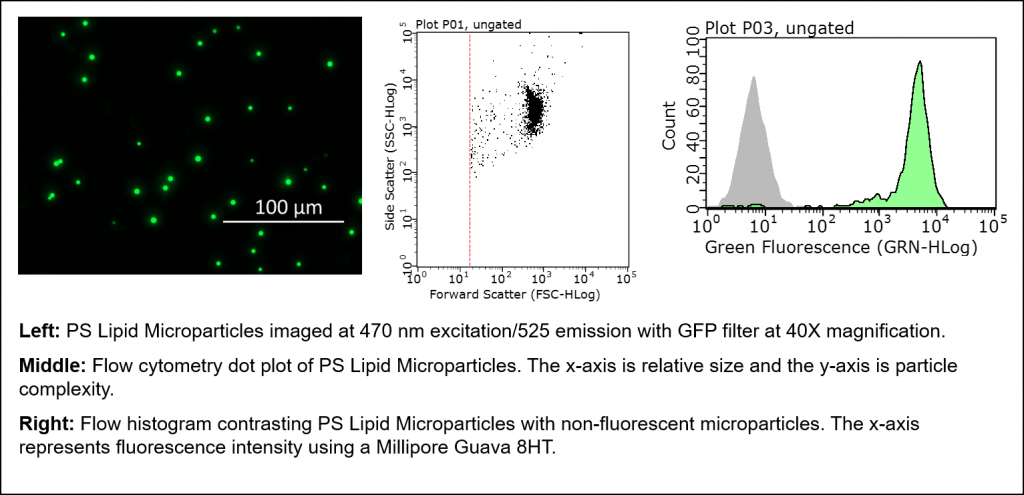
Figure 2: Examples of immunofluorescence and flow cytometry data using PS microparticles.
Microparticles as a positive control for apoptosis
A common method of measuring apoptosis in cells involves the use of the anticoagulant protein, Annexin V. This protein specificifically binds to phosphatidylserine (PS) and is utilized to measure the externalization of PS from the cellular membrane’s inner to outer leaflet. This externalization of PS to the outer membrane is indicative of a disruption of the membranes standard asymmetry and is a hallmark of apoptosis. Similarly, Annexin V can be used as a confirmatory readout of PS bound to a microparticle.
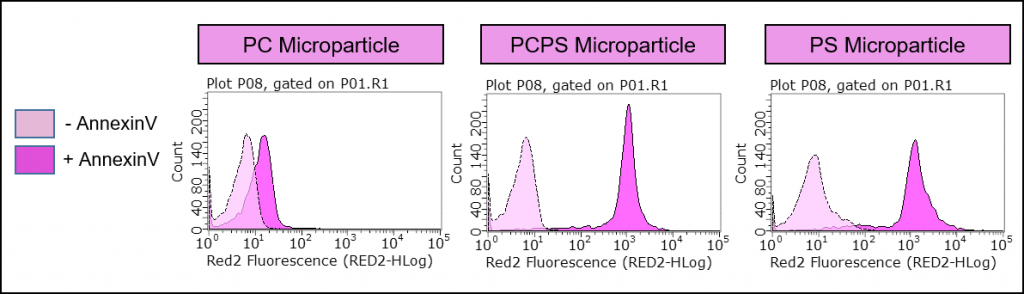
Figure 3: Specificity of Annexin V binding. Echelon’s P-B1PS and P-B1PC microparticles demonstrate preferential binding of Annexin V to our PS coated lipid microparticles, suggesting the use of this microparticle as a positive control for apoptosis analysis. The y-axis is number of events (cells) and the x-axis represents fluorescence intensity. The right-shifts of the fluorescence curves are indicative of Annexin V binding.
Lipid microparticles for use in phagocytosis studies
In early testing, we found that the P-B1PS, PS lipid microparticles, are preferentially phagocytosed at a higher percentage than the P-B1PC, PC lipid microparticles. Importantly, this data supports the use of PS Microparticles as a synthetic ‘eat me’ signal for studying apoptosis. This also suggests that the PS Microparticles can serve as a powerful research tool in the field of phagocytosis.
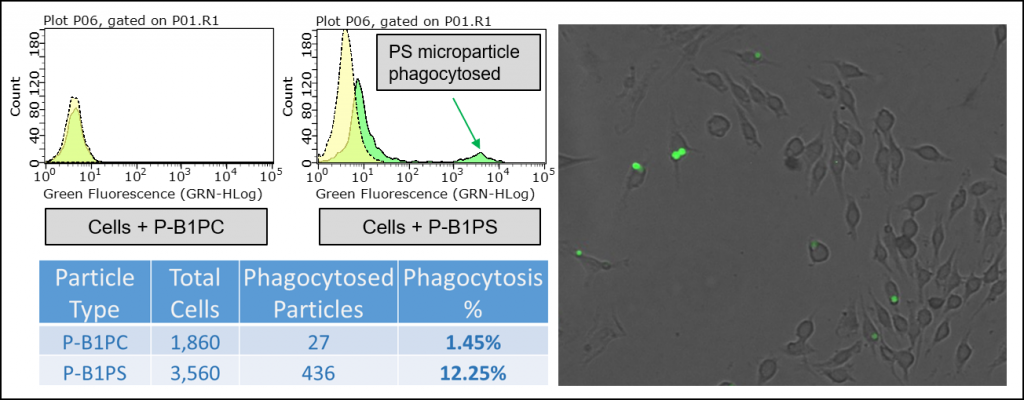
Figure 4: Top left, flow cytometry histogram contrasting PC and PS Lipid Microparticles when added to RAW 264.7 macrophage-like cell line. Cells were incubated with blocked microparticles for 0 & 45 minutes. The x-axis is fluorescence intensity. Bottom left, Table contrasting the uptake of PC vs. PS microparticles by RAW 264.7 macrophage-like cells after an incubation time of 45 minutes. Right, RAW 264.7 Macrophage-like cells treated with PS Lipid Microparticles for 45 minutes imaged at 470 nm excitation/525 emission with GFP filter at 40X magnification.
Looking ahead
Over the past 15 years, it was established that phagocytes use phosphatidylinositol phosphates (PIPs), or phosphoinositides, as signaling lipids. Specifically, PI(3)P, PI(4)P, and PI(4,5)P2 are involved in phagosome formation, trafficking, maturation, and resolution. Furthermore, there is now mounting evidence that similar PIP molecules may be involved in recognition by phagocytes of cells undergoing apoptosis. We are excited to learn more about the process of “aPIPtosis” as the details emerge.
In addition to our continued interest in the field of phagocytosis and lipid signaling, we are always excited to open up new collaborations. If we do not currently have the lipid coated product you need, we can make it! Please contact our customer service or support staff and we can work together to help answer your research questions.
Custom Microparticles
All Lipid Microparticles
1. Durrant TN, Moore SF, Bayliss AL, Jiang Y, Aitken EW, Wilson MC, et al. Identification of PtdIns(3,4)P2 effectors in human platelets using quantitative proteomics. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids. 2020
2. Zhou X, Yu J, Cheng X, Zhao B, Manyam GC, Zhang L, et al. The deubiquitinase Otub1 controls the activation of CD8+ T cells and NK cells by regulating IL-15-mediated priming. Nature Immunology. 2019
3. Kong JN, Zhu Z, Itokazu Y, Wang G, Dinkins MB, Zhong L, et al. Novel function of ceramide for regulation of mitochondrial ATP release in astrocytes. J Lipid Res. 2018
