The relationship between cancer and the immune system is complex. There is intense interest in harnessing immune cells to attack tumors and a growing need to understand the molecular details of this relationship. Macrophages are a diverse population of immune cells that may promote or inhibit tumor growth depending on their phenotype and underlying functions. Some subsets of macrophages can block tumor infiltration by supporting T-cell function or recruiting natural killer (NK) cells, while others may remodel the extracellular matrix (ECM), a driver of tumor progression. This variety of macrophage types has also been shown within the tumor-microenvironment, therefore understanding the functions of individual macrophage types could be beneficial for therapeutic development.
In the current study, Elfstrum and colleagues investigate a macrophage type expressing LYVE-1. LYVE-1 is a transmembrane protein that acts as a receptor for hyaluronic acid (HA). LYVE-1+ macrophages normally regulate tissue homeostasis, however, they have also been associated with breast cancer and appear to drive the formation of new lymphatic vessels in tumors in some cancer models. Here, using Echelon's HA ELISA (Echelon, K-1200), the researchers show that LYVE-1+ macrophages regulate HA turnover and HA accumulates in mammary tissue in a genetic mouse model where these cells are depleted. In this model, ECM remodeling is also altered, leading to reduced mammary tumor growth in vivo. Subsequent experiments also show that LYVE-1+ macrophages have higher hyaluronidase activity (Echelon, K-6000) which drives their ability to remodel the ECM. Furthermore, RNA-seq analysis of macrophages from animals where LYVE-1+ cells where depleted show that the remaining macrophage populations adopt a proinflammatory state and may recruit more T cells to tumor sites.
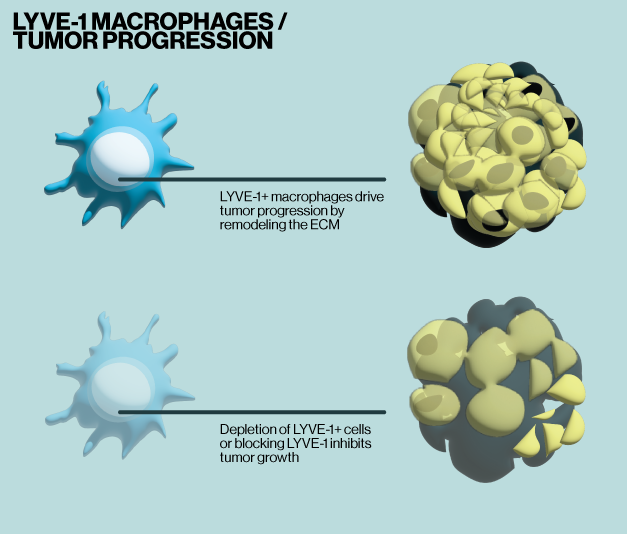
These results demonstrate that the LYVE-1+ macrophage population maintains an anti-inflammatory tumor microenvironment, supports tumor growth, and restricts T-cell count. Together, these findings indicate that LYVE-1+ macrophages are a type of tumor-promoting macrophage that degrade HA thereby remodeling the ECM in the tumor microenvironment. These findings also point to a few potential mechanisms that could be harnessed for therapeutic benefit: 1) blocking LYVE-1 macrophage binding to HA 2) selective depletion of LYVE-1+ macrophages 3) tuning activity of hyaluronidases and HA synthases within the tumor microenvironment. While targeting macrophages clinically remains challenging, the current work highlights their importance to tumor progression and provides new routes to improved therapeutics.
Read the full article here:
Cancer Research Communications (2024)
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences