Methods for determining Hyaluronic Acid molecular weight
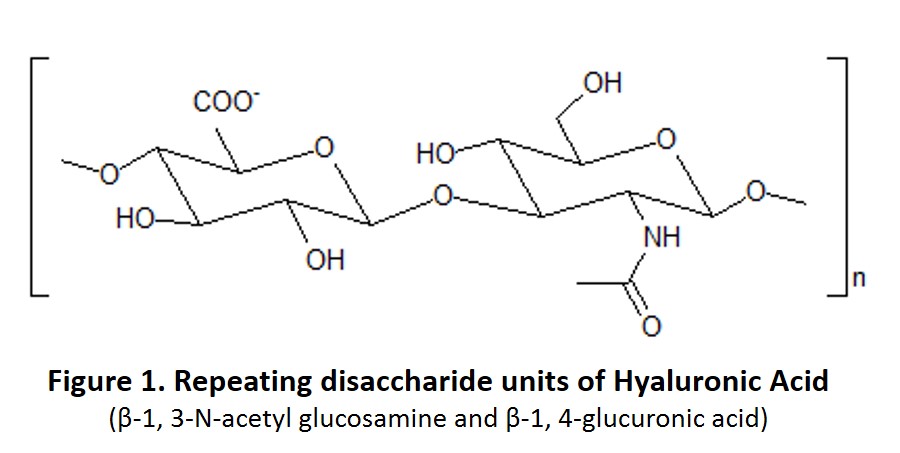
Hyaluronic Acid, also known as Hyaluronan, is a natural non-sulfated glycosaminoglycan produced in many organs and tissues (Figure 1). Hyaluronic Acid was discovered in the 1930s and was originally thought to have no physiological function except serving as a lubrication “space filler” between joints.
With additional research, hyaluronic acid is now appreciated as an important part of the extracellular matrix. Indeed, it has critical roles in many cell signaling events such as proliferation, inflammation, wound healing, and fertilization. Furthermore, both hyaluronic acid concentration and its molecular weight changes in certain diseases such as osteoarthritis1,2.
While methods for measuring hyaluronic acid concentration are well established (ELISA), measuring hyaluronic acid molecular weight is much more complicated. Three methods have been widely used to determine hyaluronic acid molecular weight: viscometry, conventional size exclusion chromatography (conventional-SEC), and size exclusion chromatography with multi-angle laser light scattering detector (SEC-MALLS). Viscometry is labor intensive and SEC requires sophisticated, high-cost equipment. All three methods present the potential for errors in hyaluronic acid molecular weight estimation3. Using monodisperse hyaluronic acid standards, hyaluronic acid molecular weight can now be determined using a common molecular biology technique – gel electrophoresis.
So, how do you determine hyaluronic acid molecular weight using gel electrophoresis? You can find the detailed protocol for hyaluronic acid gel electrophoresis here.
Below are 3 tips to ensure a perfect hyaluronic acid gel.
1. Choose the Right Reagents
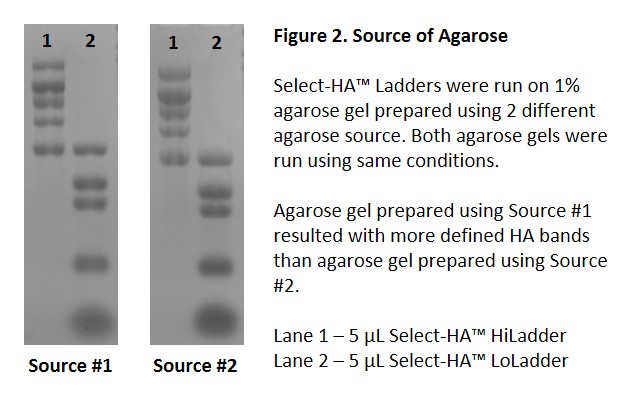
It has been reported that the source of the agarose significantly affects the gel resolution and staining background4. Data from Echelon further support these findings. We found using a high purity agarose is critical (Figure 2).
Buffer choice is also important. While borate-based buffer provides a tighter matrix for higher resolution, acetate-based buffer is preferred when running hyaluronic acid samples with size greater than 4000 kDa. However, buffer exhaustion occurs in acetate-based buffer. Therefore, small agarose gel with large electrophoretic chamber should be used in order to maintain pH when the acetate-based buffer is the buffer of choice. The recommended gel running conditions are summarized in Table 1.
2. Be Patient!
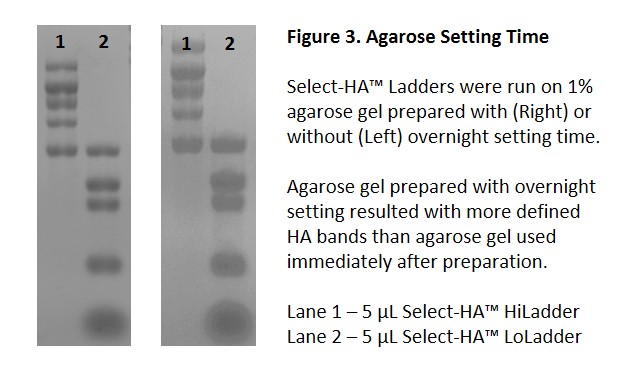
Although borate-based buffer provides better gel resolution, gel setting time is also important for good resolution. Letting agarose gel set overnight shows significant improvement on resolution when compared to an agarose gel run immediately after preparation (Figure 3).
In addition, low voltage is also important for a good resolution, especially for high molecular weight hyaluronic acid samples. So, be patient and let the agarose gel run at a lower voltage for a little longer. Finally, ensure the salt concentration of your hyaluronic acid sample is low and the sample is fully re-hydrated. High salt and insufficient hydration will affect your hyaluronic acid sample migration during electrophoresis. Desalt your sample and let your sample re-hydrate overnight if necessary.
3. Know Your Samples
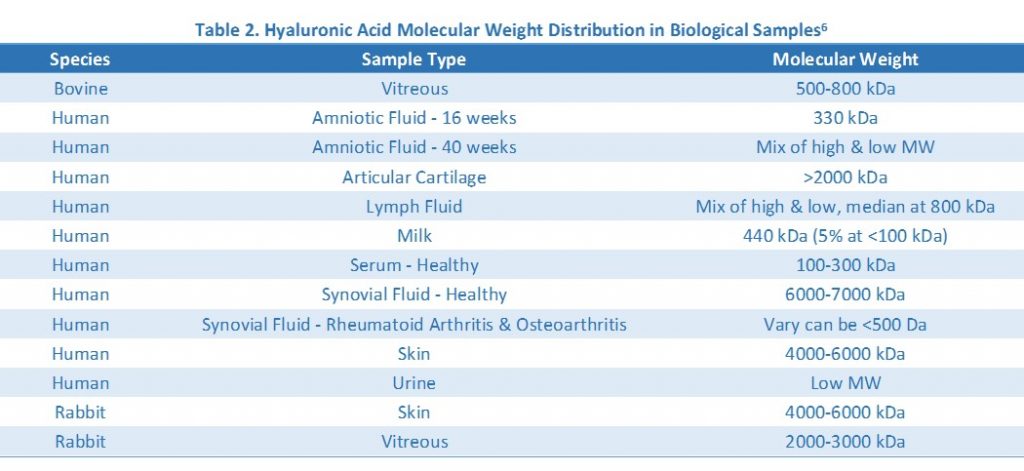
While the goal of the gel electrophoresis is to determine the molecular weight of your hyaluronic acid samples, knowing your sample concentration and the estimated molecular weight would be very helpful in determining how much sample to load on the gel and which gel matrix to use. You can easily measure your sample’s hyaluronic acid concentration by using the Hyaluronic Acid ELISA. Hyaluronic acid molecular weight distribution in common biological samples is summarized in Table 2.
We hope these tips are helpful to pursue your hyaluronic acid research and experiments. If you need help in selecting the correct products, contact us here. Good luck with your project!
Check out Echelon’s full Hyaluronic Acid product line here
References
1. Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Fujita, H.; Takahashi, I.; Umeda, T.; Nakaji, S.; Ishibashi, Y., Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis – a five-year prospective cohort study. Arthritis Res Ther 2015, 17, 283.
2. Band, P. A.; Heeter, J.; Wisniewski, H. G.; Liublinska, V.; Pattanayak, C. W.; Karia, R. J.; Stabler, T.; Balazs, E. A.; Kraus, V. B., Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthritis Cartilage 2015, 23 (1), 70-6.
3. Shanmuga Doss, S.; Bhatt, N. P.; Jayaraman, G., Improving the accuracy of hyaluronic acid molecular weight estimation by conventional size exclusion chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 2017, 1060, 255-261.
4. Bhilocha, S.; Amin, R.; Pandya, M.; Yuan, H.; Tank, M.; LoBello, J.; Shytuhina, A.; Wang, W.; Wisniewski, H. G.; de la Motte, C.; Cowman, M. K., Agarose and polyacrylamide gel electrophoresis methods for molecular mass analysis of 5- to 500-kDa hyaluronan. Anal Biochem 2011, 417 (1), 41-9.
5. Cowman, M. K.; Chen, C. C.; Pandya, M.; Yuan, H.; Ramkishun, D.; LoBello, J.; Bhilocha, S.; Russell-Puleri, S.; Skendaj, E.; Mijovic, J.; Jing, W., Improved agarose gel electrophoresis method and molecular mass calculation for high molecular mass hyaluronan. Anal Biochem 2011, 417 (1), 50-6.
6. Cowman, M. K.; Lee, H. G.; Schwertfeger, K. L.; McCarthy, J. B.; Turley, E. A., The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front Immunol 2015, 6, 261.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences