In the realm of biological research, the efficient delivery of molecules into cells is a fundamental yet often challenging task. Some methods are laborious, require specialized equipment, or can be damaging to cells. Echelon Biosciences has pioneered a novel approach for delivering phosphatidylinositol phosphate (PIP) lipids with our Shuttle PIP products. These lipids are part of a complex set of signaling cascades in the cell and can be difficult to study in isolation. Our innovative tools offer a simple, efficient, and non-toxic method for delivering PIPs into a wide range of cell types, which allows for the cellular localization and effects of PIPs to be studied without having to activate their associated synthetic pathways in the cell.
The Power of Shuttle PIP Technology
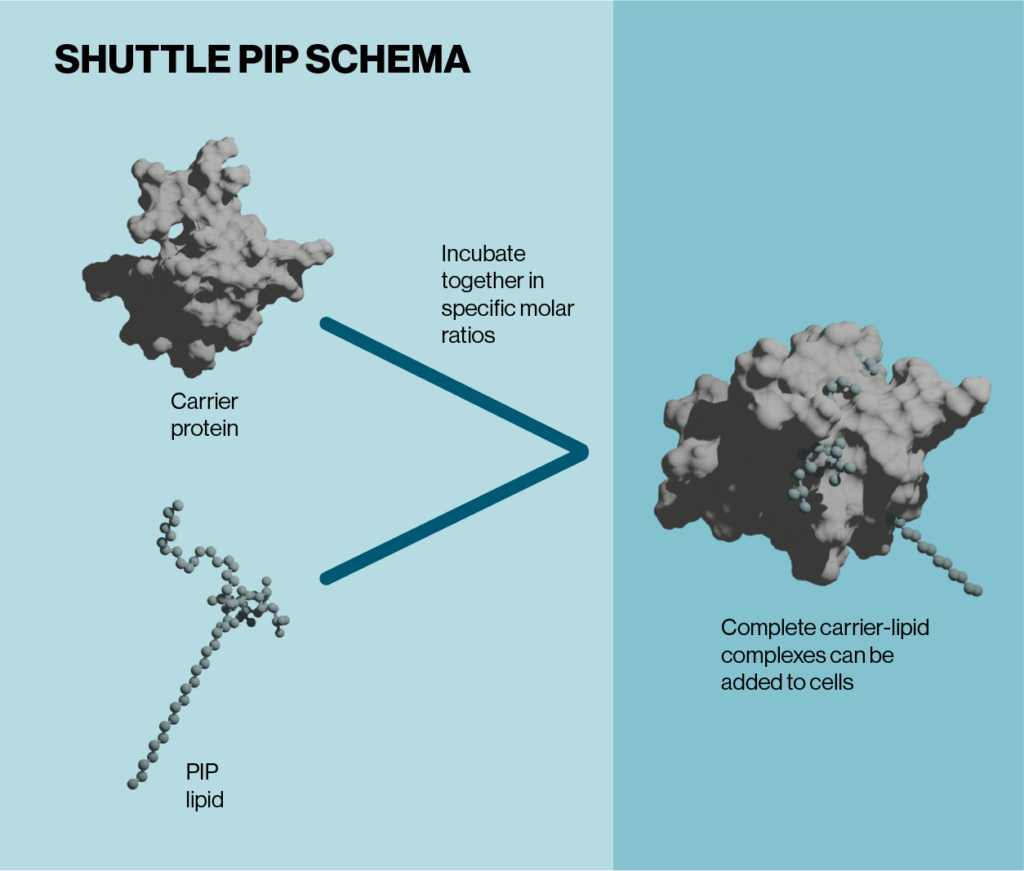
Shuttle PIPs are primarily designed for convenience. Simple carrier molecules, derived from small molecules or proteins, are mixed with the desired PIP and incubated briefly for 5-15 minutes (see figure below). The resulting lipid-carrier complexes that are formed can then be diluted and added directly to cultured cells or cell media. Each Shuttle PIP kit contains a fluorophore-conjugated carrier or PIP, which enables a visible readout of when the lipid-carrier complexes have been taken up by cells. The high efficiency of the delivery mechanism means that the technology can be applied to multiple cell types including adherent or suspension cells or even more delicate and complex culture systems such as primary cells and organoids. Additionally, unlike other cell delivery methods and transfection reagents, the kit components are non-toxic meaning no further manipulation is required after addition of the lipid-carrier complexes to cells. Compatibility with a variety of culture systems makes Shuttle PIPs an extremely versatile tool for studying a wide range of biological processes.
Applications
Shuttle PIP technology has found uses in a wide array of biological research areas. Recent work that has utilized these reagents ranges from protein function to host-pathogen interactions. One example involves a protein named interferon-induced transmembrane protein 3 (IFITM3), an antiviral protein that functions in the endosomal compartment. However, the IFITM3 gene has also been associated with certain cancers, including leukemia and lymphoma.
Initial experiments by Lee et al. found that phosphorylation of IFITM3 causes it to localize to the plasma membrane and that oncogenic kinases cause this localization to be constitutive. At the membrane, IFITM3 engages with other proteins that mediate downstream PI3K signaling and deletion of ITITM3 in B cells resulted in a loss of receptors associated with lipid rafts in the cell membrane. Additionally, IFITM3 bound selectively to PI(3,4,5)P3 (PIP3), the lipid product of PI3K activity, which facilitated membrane localization. The authors used Shuttle PIPs (P-9C2, P-3916) to deliver PIP3 to cells to separate the membrane functions of ITITM3 from downstream PI3K activity in ITITM3 deficient cells. They found that adding PIP3 to B cells rescued PI3K signaling but did not restore clustering of receptors into lipid rafts. Their data demonstrate that ITITM3 affects PI3K at multiple levels and how constitutive activation of this protein can lead to malignant cells.
A similar depletion-and-rescue experimental structure with Shuttle PIPs was used by Matsumoto et al. to interrogate the cellular and molecular mechanisms of cell division in a plant model. Here, the authors proposed that a plant SNARE protein, VTI11 is involved in a form of membrane fusion that is dependent on a phospholipid synthesis pathway. After establishing that vti11 mutants were deficient in membrane fusion, the researchers investigated the requirement for specific phospholipids. They first used a small molecule inhibitor to deplete the plant cells of PI(3)P which rescued defects associated with vti11 mutants. Addition of PI(3)P via Shuttle PIPs (P-3008, P-9C3) after inhibitor treatment restored the mutant defects. In this manner, they were able to show that the proper SNARE-membrane functions of VTI11 are at least partially dependent on the presence of PI(3)P in the membrane.
Lastly, Shuttle PIPs have also been used to dissect more complex cellular events such as pyroptosis, a type of inflammatory cell death. Pyroptosis is dependent on a family of proteins called gasdermins (GSDM) that form small pores in cell membranes that facilitate the release of cytokines for a larger inflammatory cascade. This mechanism is important for controlling pathogens. Among GSDM proteins, GSDM-D is the most well studied. The pore-forming funcions of GSDM-D are dependent on proper membrane localization. Here, Chai et al. show that pathogens such as M. tuberculosis secrete phospholipase proteins that dephosphorylate PI(4)P and PI(4,5)P2 (PIP2). This results in a loss of GSDM-D at the plasma membrane and defective pyroptosis. The authors delivered PIP2 (P-9045) to cells in the presence of M. tuberculosis and were able to restore membrane localization of GSDM-D and pyroptosis. Thus they were able to demonstrate the complex interaction of a cell’s inflammatory response to a pathogen and parallel actions from the pathogen to evade this response.
Summary
These examples demonstrate the effectiveness of Shuttle PIP products across several cellular applications. Their simplicity, efficiency, and versatility make them an invaluable tool for researchers in a variety of fields. By providing a reliable and user-friendly method for delivering PIPs into cells, Shuttle PIPs are a significant advancement in cell delivery technology and help accelerate scientific discovery.
Citations
1. Lee, J., et al., IFITM3 functions as a PIP3 scaffold to amplify PI3K signalling in B cells. Nature, 2020.
2. Chai, Q., et al., A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science, 2022. 378(6616): p. eabq0132.
3. Matsumoto, H., et al., Dynamic Rearrangement and Directional Migration of Tubular Vacuoles are Required for the Asymmetric Division of the Arabidopsis Zygote. Plant and Cell Physiology, 2021.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences