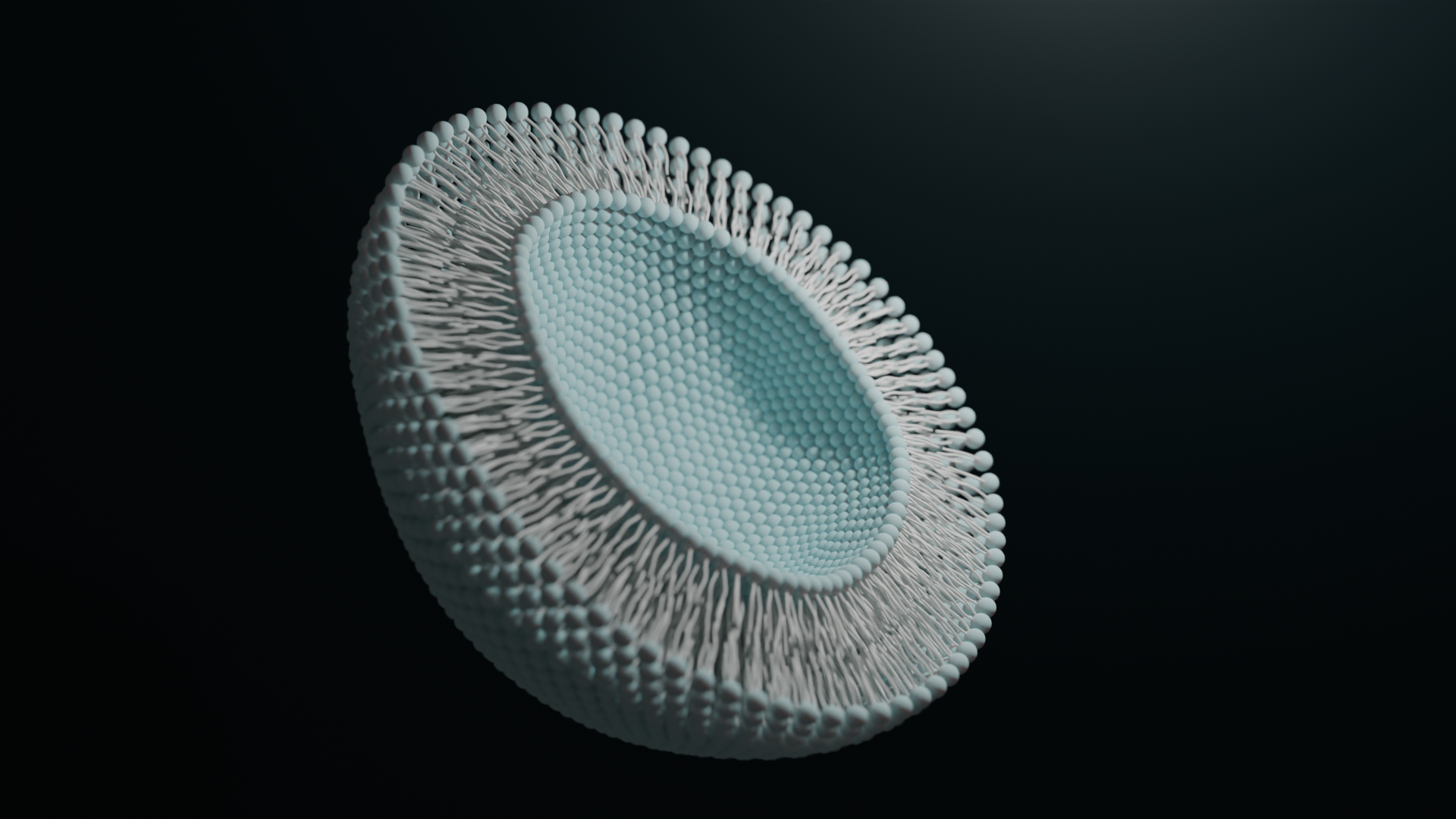
Echelon’s Lipid Nanoparticle Services include formulation, RNA synthesis, and expression screening in cell culture. Utilize our combined expertise in cell biology and lipid chemistry to help select ionizable lipids from our catalog to optimize LNP formation with your cargo. We can also synthesize mRNA from your custom DNA templates to minimize turnaround time for your final LNP product.
Formulation
Our formulation services are available for all of the ionizable lipids available in our product catalog. We will optimize your RNA cargo for packaging with a single or multiple ionizable lipids, and our team can help guide your selection of which lipid to use. Synthesis of unique, novel ionizable lipids is also available. Once robust LNP formation is achieved, a standard set of analyses is run as part of our quality assurance, including encapsulation efficiency and polydispersity.
mRNA Synthesis
In addition to LNP Formulation, we offer In Vitro Transcription (IVT) for production of mRNA cargo. mRNA is co-transcriptionally capped to enhance stability and downstream expression. IVT with uridine analogs such as pseudouridine is also available. This has been demonstrated to reduce immunogenicity of mRNA cargos and may be required for more sensitive cell targets such as immune derived cells. Downstream purification of resulting transcripts can be performed by gel or anion exchange according to the scale and needs of the project.
Expression Screening
Unsure about testing your LNP in a cell or animal model? We can help! We can prescreen your LNP for expression using any of our available cell lines. This may be done utilizing tags or antibodies directed at your cargo of interest or by co-delivery of a mRNA for a fluorescent protein. We can also incorporate fluorescently conjugated lipids in the LNP formulation in order to differentiate between insufficient LNP delivery or cargo expression. Our cell biology experts will guide you through the process to get the best results.
Other considerations
Selecting an ionizable lipid: Selection of an appropriate ionizable lipid is based on how well the cargo is packaged and the target cell or tissue type for delivery. Successful encapsulation and delivery with a specific ionizable lipid and a generic mRNA such as GFP or Luciferase may not directly correlate with a custom therapeutic mRNA.
IVT parameters: We mainly utilize the T7 RNA polymerase for IVT. As such, a T7 promoter site is required for any DNA template provided for the service. Promoter sequences should also be checked for elements that confer addition of cap analogs as different commercially available capping reagents may have different sequence requirements.
Ready to get started on your project? Fill out the form below!
0.2
/ 0.2