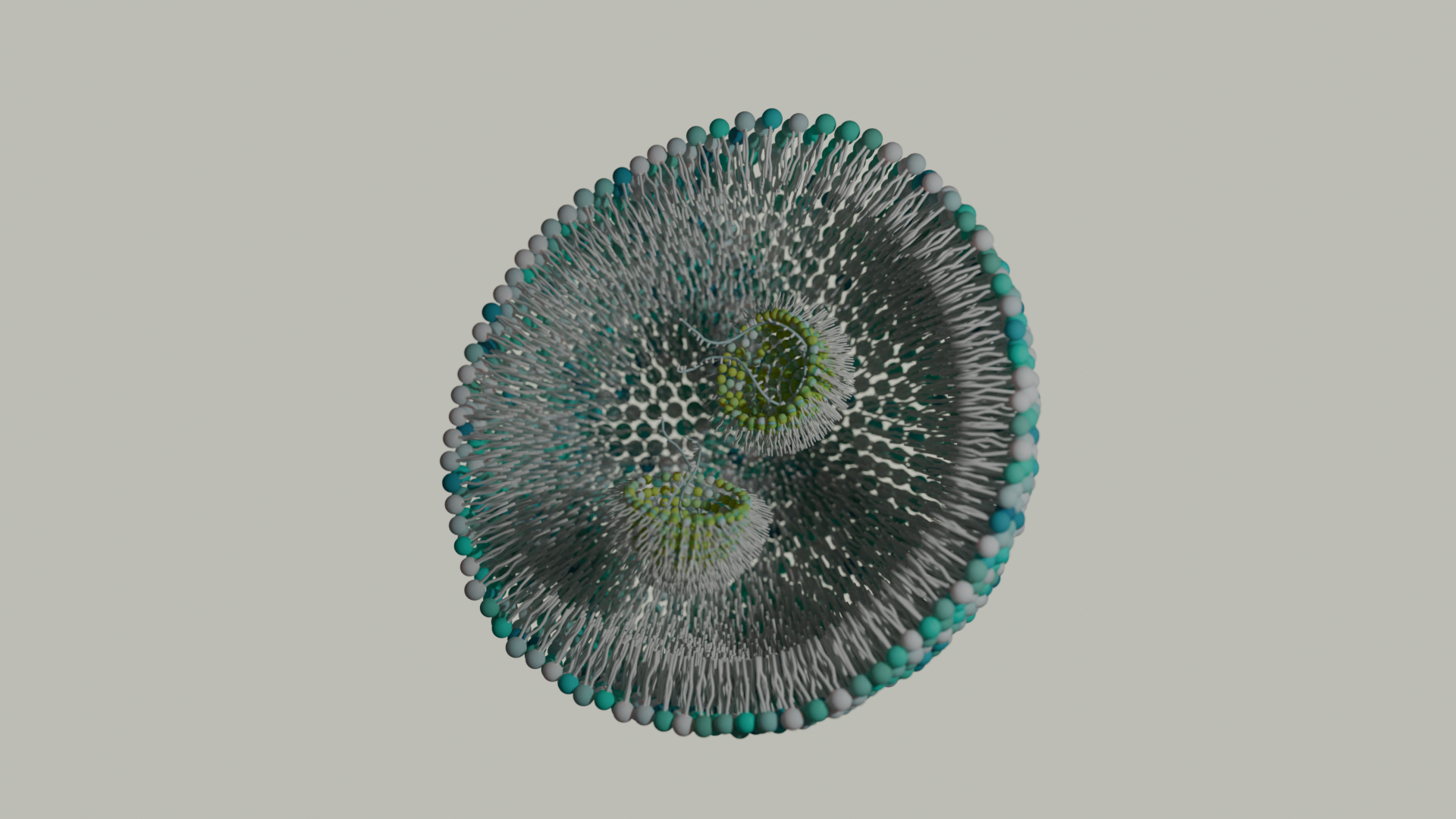
Genetic therapies have now been pursued for several decades and close to a dozen DNA-based gene therapies have gained regulatory approval in the last decade. Despite these recent successes, DNA gene therapies remain limited as the majority of them use viral vectors that can cause unwanted immune activation in patients. Alternative therapeutics based on RNA could hypothetically bypass some of the issues associated with DNA gene therapies and have also been the focus of intense research and development. Furthermore, the approval and effectiveness of the first RNA medication and mRNA-based vaccines within the last 5 years has only heightened the interest in these types of therapies. The main drawback to using RNA as a therapy is naked RNA cannot simply be injected as it is immunogenic, easily susceptible to enzymatic degradation, and is not taken up by cells. Therefore, alternative delivery systems are required for delivering therapeutic RNA. Currently approved RNA therapeutics employ lipid nanoparticles (LNPs) to overcome these problems. RNA is packaged up in LNPs that protect it from degradation while circulating, allow it to enter cells, then release the contents into the cytoplasm so the RNA can be used by ribosomes to synthesize the desired protein(s). The key component of RNA-LNPs are ionizable lipids. These lipids bind RNA allowing it to be encapsulated into the liposome-like LNPs.
LNP Formulation
Decades of research have gone into developing and formulating the lipids that make up effective LNPs. Essentially, 4 types of lipids are required to formulate LNPs (approximate proportions in brackets):
- Ionizable Lipid. This is the key component of the LNP [35-50%] and has two main roles: bind the RNA and allow release of the RNA in the cell. The pKa of the lipid is an important factor as the lipid needs to be positively charged at low pH in the LNP to bind RNA but uncharged at neutral pH so the LNP does not cause toxicity. Hundreds of lipids were synthesized and screened by many research groups to identify those with the desired properties and effects. Some successful candidates include ALC-0315, cKK-E12, SM-102, and Dlin-MC3-DMA.
- PEGylated lipid. A small amount of a PEG derivatized lipid [0.5-3%] is incorporated to increase the circulatory half-life in the body. PEG-lipids have also been used for years in liposomal drug delivery systems creating so-called “stealth” liposomes. In addition, the percentage of PEG-lipid influences the size of the LNP. Examples include ALC-0159, DSPE-mPEG, and DMG-mPEG.
- Cholesterol. Cholesterol is a structural “helper” lipid that makes up a significant part of the LNP [40-50%] and improves efficacy possibly by promoting membrane fusion and promoting endosomal escape.
- Neutral phospholipid. Synthetic phospholipids such as DSPC, DPPC, and DOPE [~10%] are also commonly used as structural “helper” lipids in LNP formulation to promote cell binding.
To manufacture RNA-containing LNPs, the lipids in ethanol and the RNA in low pH buffer are rapidly mixed in a microfluidic mixer. The ionizable lipid is protonated and begins to bind and encapsulate the RNA. The pH is gradually increased to 7.4 and the ethanol is removed, completing the formation of the LNPs (Figure 1). The efficiency of the RNA encapsulation is quite high using microfluidic mixing compared with traditional extrusion used for liposomes. The characteristics of the LNP formulation such as particle size, surface charge, tissue targeting, etc. can be tailored by the type and ratio of lipids in the LNP.
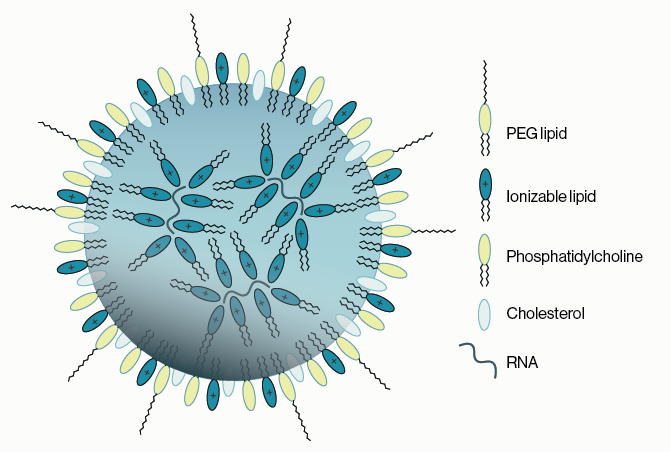
Figure 1: An example of the components of a lipid nanoparticle (LNP). An outer lipid coat composed of ionizable lipids, PEGylated lipids, cholesterol, and helper phospholipids surrounds a core of ionizable lipids encapsulating the RNA cargo.
After the LNPs are injected, the particles are taken up by cells in endosomes. The pH decreases during endosomal maturation protonating the ionizable lipids and causing the endosomes to burst, releasing the LNPs into the cytosol. The higher pH of the cytosol triggers the LNPs to dissociate, releasing the RNA for ribosomal translation and protein expression to produce the desired effect.
Current Outlook
The main limitation with current LNP technology is site or cell specific targeting. The first FDA-approved LNP drug, Onpattro, was shown to accumulate within the liver in hepatocytes. In this specific case, this was advantageous as the protein being targeted for therapy is synthesized in the liver. However, delivery to hepatocytes was found to be associated with circulating ApoE binding to the LNPs. As ApoE receptors are predominantly expressed in hepatocytes this explains the enrichment in the liver, however this also indicates that hepatic accumulation would occur with any systemically administered LNP. Therefore, for any LNP therapy that requires delivery to a specific organ or cell population, alternative delivery and targeting technologies are needed.
There are three main strategies for site specific targeting of LNPs: 1) localized delivery 2) addition of targeting ligands 3) inclusion of modified lipids. Localized delivery has already been shown to efficacious with the development of recent mRNA vaccines. In these cases, localized intramuscular injection is sufficient to stimulate a systemic immune response while avoiding LNP accumulation in the liver. This type of delivery may be sufficient for other organs or tissue that are easily accessibly by injection. A second approach is to incorporate a membrane associated protein or peptide ligand with a receptor that is enriched in or selective for certain cell types. This is similar to the approach used for such therapies as CAR-T and while this would not fully eliminate hepatic accumulation, it could be sufficient to enhance tissue specific uptake. Lastly, the use of synthetic lipids could also bias delivery away from the liver. There are several different forms this scheme could take, but the two simplest approaches are incorporating a lipid with a charged headgroup to alter LNP surface chemistry or using a lipid-ligand conjugate to target LNPs. Changing LNP surface chemistry could potentially reduce non-specific interactions with hepatically enriched proteins thus making LNPs more bioavailable. The use of a lipid-ligand conjugate is similar to the incorporation of a protein or peptide ligand with the benefit that small molecule ligands could potentially be conjugated to the lipid headgroup.
In order to expand the possible applications for LNPs, it is probable that all three of these strategies will need to be pursued and perhaps implemented within a single LNP formulation. In all of these cases, new ionizable lipids will likely be required and with them the ability to quickly screen for efficacy.
References
1. Buschman MD, Carrasco, MJ, et al. (2021) “Nanomaterial Delivery Systems for mRNA Vaccines” Vaccines 9:65
2. Tenchov, R, Bird, R, et al. “Lipid Nanoparticles – From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement” ACS Nano DOI:10.1021/acsnano.1c04996
3. Guevara ML, Persano F and Persano S (2020) “Advances in Lipid Nanoparticles for mRNA-Based Cancer Immunotherapy.” Front. Chem. 8:589959. doi: 10.3389/fchem.2020.589959
4. Recent Advances in Site-Specific Lipid Nanoparticles for mRNA Delivery PMID: 37360845
5. Current state of RNA delivery using lipid nanoparticles to extrahepatic tissues: A review towards clinical translation PMID: 37276899
6. An ionizable lipid toolbox for RNA delivery PMID: 34903741
7. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection PMID: 32832671
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences