Most molecular and cell biologists have an array of techniques in their toolkit that make use of conventional IgG or IgM antibodies (Abs). Custom antibody production has become a mainstay for biology techniques such as immunofluorescence and western blotting. Less familiar are the second-generation antibodies which are engineered smaller versions of antibodies that maintain specific binding to target antigens (proteins, nucleic acids, lipids, carbohydrates). Two examples are antigen-binding fragment (Fab) and single-chain variable fragment (scFv). These second-gen antibodies have been very valuable in certain research applications, but they have limited clinical utility as therapeutics due to issues with poor circulating half-life.
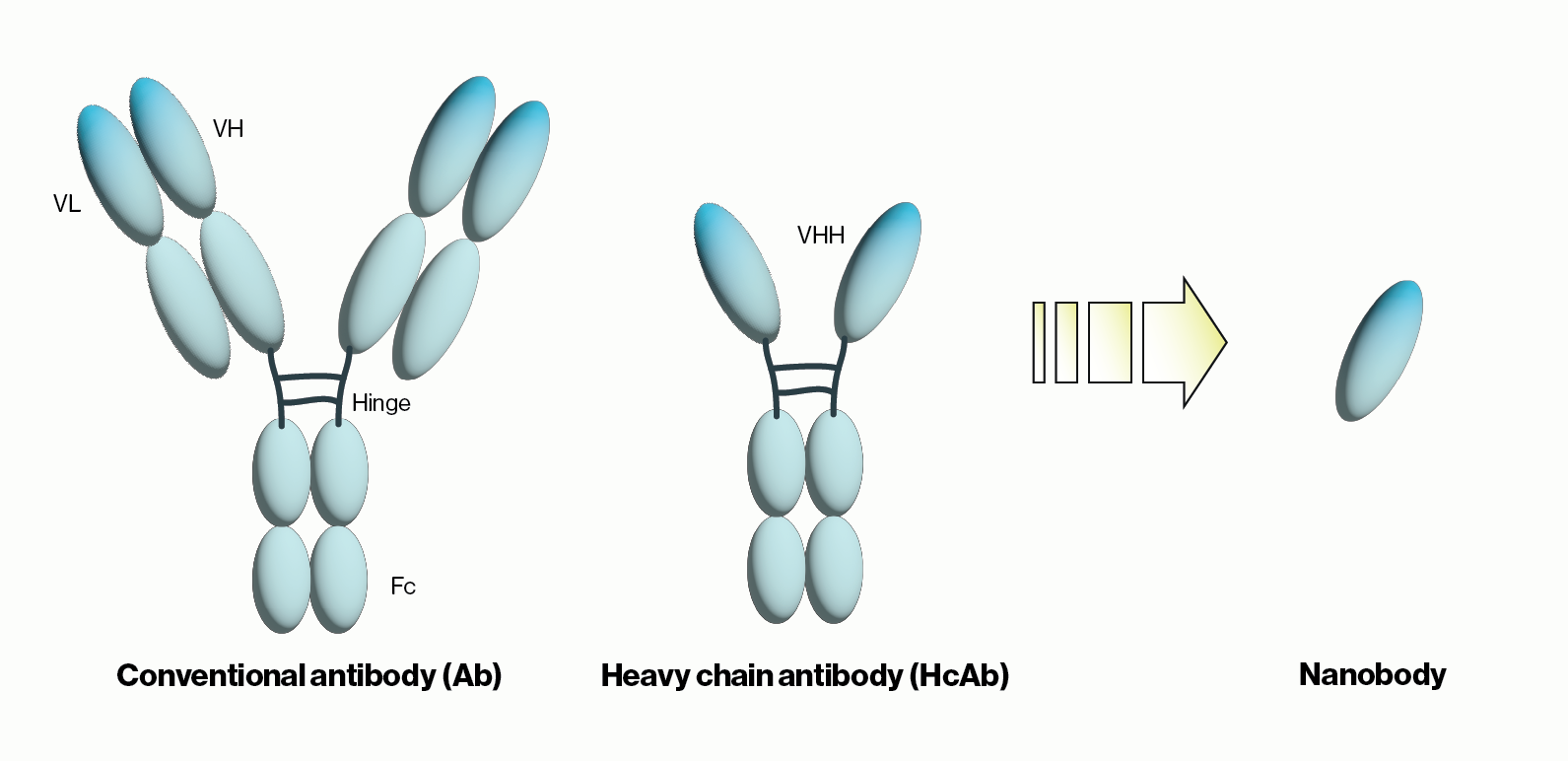
Another recent development was the unexpected discovery of heavy chain-only antibodies (HcAbs) in sharks and camelids. A typical antibody, as exemplified by human IgG, contains two 50 kDa heavy chains and two 25 kDa light chains for a combined molecular weight of 150 kDa. Conversely, camelid HcAbs are made up of two single variable domain heavy chains giving them a size that is considerably smaller than conventional antibodies. Importantly, the single variable domain (VHH) from HcAbs can be isolated and maintains the binding properties observed with the full HcAbs. The resulting VHH antibodies were named ‘nanobodies’ due to their low molecular weight of approximately 15 kDa. Below we will briefly summarize nanobody applications and some of the advantages they provide over traditional antibodies.
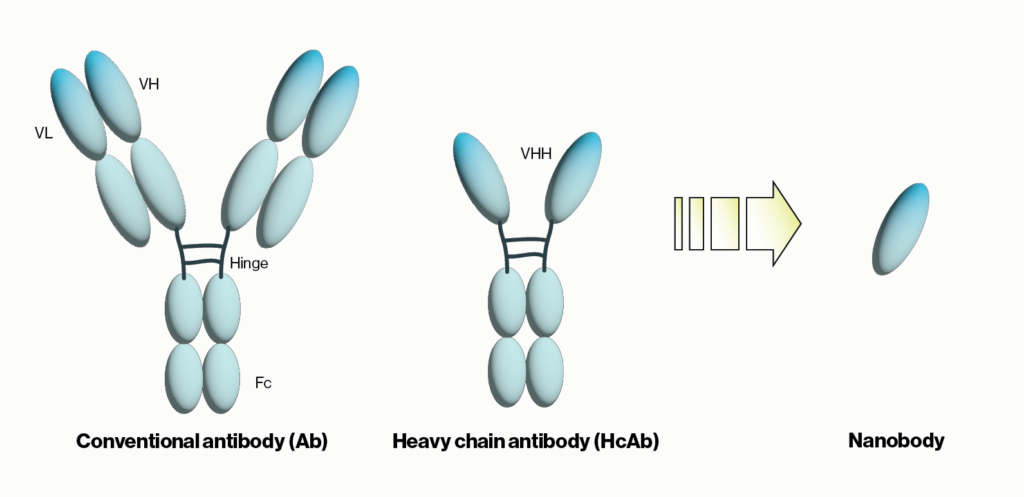
Figure 1: Schematic comparison of conventional antibodies, heavy chain antibodies, and nanobodies. VH = heavy chain variable domain, VL = light chain variable domain, VHH = single variable domain heavy chain. IgG Ab are ~150 kDa in size while HcAb are 50 kDa and their corresponding nanobodies are 15 kDa.
Nanobody production
Generating a target specific nanobody begins similarly to traditional antibody production by immunizing an animal, in this case a llama or alpaca, with an antigen of interest. Immune cells containing the HcAbs of interest are then harvested from a simple blood draw from the animal. Because the cDNAs of the VHH are well characterized, mRNA from these cells can be isolated and PCR amplified to generate sequences for the VHH only. These can then be cloned and screened by phage display with resulting hits transferred to bacterial or mammalian expression vectors for larger scale production. Once the DNA sequences from these hits have been identified and cloned, the resulting constructs enable further manipulation such as the addition of affinity tags or cellular targeting sequences. The availability of cDNAs for a given nanobody also removes barriers or concerns about lot-to-lot variability that can arise with polyclonal antibodies. Learn more about custom nanobody production here:
Applications
As with conventional Abs, nanobodies can generally be utilized for long standing methods such as western blotting, ELISAs, and immunofluorescence. Where nanobodies have a distinct advantage is in the realm of cellular and in vivo imaging. Their size makes them ideal tools for super-resolution microscopy (super-res). Depending on the methodology individual protein particles in cells can typically be localized to 20-50nm with super-res. However, the use of paired primary-secondary Abs or labeled primary antibodies may increase the lower limit of resolution due to size constraints. One solution is to produce a labeled fusion gene within the cells or tissue, but this obviously requires either direct manipulation of the endogenous genome or overexpression of the gene-of-interest. An attractive alternative is to use a labeled nanobody introduced by transfection or applied by cytochemical staining. In either case, nanobodies overcome the size constraints of traditional Abs and improves resolution.
Owing to their small size and structure, nanobodies can bind molecular structures and penetrate tissues that are sterically inaccessible by larger Abs. This allows for visualization of previously difficult to image areas or structures. This feature is particularly advantageous for in vivo imaging as nanobodies can access deeper layers of solid tumors and cross the blood-brain barrier. Targeting a nanobody for a specific tissue or tumor marker allows for more precise imaging and labeling (without radioactive isotopes) to simplify non-invasive techniques that have previously relied on either invasive or imprecise imaging modalities.
The small size of nanobodies also make them amenable to binding cavity-like regions of proteins. Unlike Abs, the antigen-binding region of nanobodies tends to have a longer, protruding shape. This enables them to bind the active sites of enzymes or to the ligand-binding sites of cell receptors. This property opens up new opportunities to study enzyme and receptor activities in intact cells without the need for genetic manipulation of the targets.
Possible Therapeutics?
For nearly 50 years, traditional antibodies have been pursued for their therapeutic potential. To date, there are more than 60 FDA approved monoclonal Abs for various diseases, though the success rate for clinically tested Abs remains low. One challenge is that traditional Abs tend to aggregate in circulating blood, likely due to cell receptors that bind heavy chains as well as large hydrophobic patches that are present on their surfaces. This can severely limit their bioavailability and efficacy. Conversely, nanobodies are comparatively hydrophilic, do not contain regions that bind Fc receptors, and do not denature easily. This makes them compelling candidates for next-generation antibody therapies that target specific tumor or other disease-related antigens.
Currently there are roughly a dozen nanobody therapies in some stage of clinical testing. It appears likely that as the breadth of nanobody targets is expanded, their utility as diagnostics or therapeutics will grow as well. Interest in small molecule nanobody targets is emerging which could radically increase the utility of nanobodies as research reagents and beyond into drug discovery.
Find our Nanobody products here
References
1. de Marco A (2020) Recombinant expression of nanobodies and nanobody-derived immunoreagents. Prot. Exp. Pur. 172:105645
2. Yang E, Shah K (2020) Nanobodies: Next Generation of Cancer Diagnostics. Front. Oncol. 10:1182
3. Rashidian M, Ploegh H (2020) Nanobodies as non-invasive imaging tools. Immuno-Onco Tech 7:2-14
4. Cromie K, Van Keeke G, Boutton C (2015) Nanobodies and their Use in GPCR Drug Discovery. Curr Top Med Chem. 15(24):2543-57
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences