The concept of biological, intracellular phase separation has existed for some time. However, until recently direct evidence for this phenomenon was limited, largely due to technical constraints. As imaging techniques have advanced, it has become easier to observe and manipulate phase-separated compartments within the cell, although there is still much to be determined regarding their functions.
The current study from Dumelie and colleagues discusses the presence and content of non-membranous organelles, called biomolecular condensates, within cells. These condensates, such as nucleoli, nuclear speckles, and stress granules, phase separate from the nucleoplasm or cytoplasm and generally form through weak interactions between proteins and RNA. Surprisingly, there is also evidence that metabolic enzymes, particularly those involved in phospholipid metabolism, are found in these condensates. This suggests that these condensates may contain phospholipid substrates that traditionally reside in the plasma membrane or membranes of other cellular organelles.
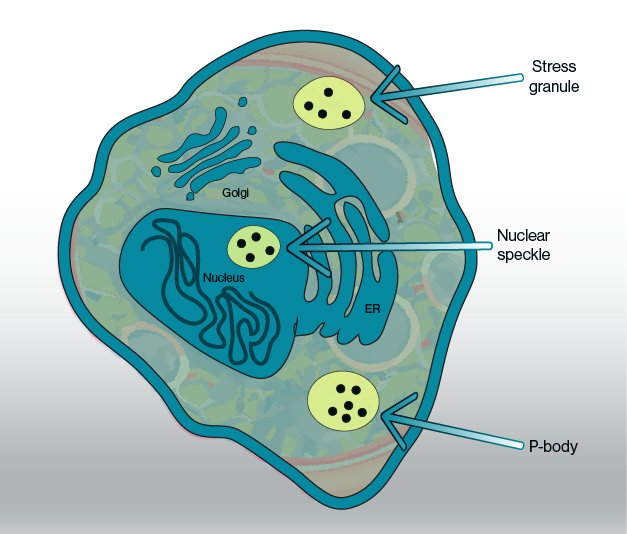
Figure 1: Putative phase-separated condensates and their location inside the cell. Current evidence points towards some subcelluar organelles such as stress granules, nuclear speckles, and P-bodies as biomolecular condensates with specific metabolite pools.
Here, the researchers hypothesized that intracellular metabolites, including phospholipids, may be selectively enriched in condensates. Experimental findings using untargeted metabolomic analysis support this notion, revealing condensate-specific metabolomes. Phospholipids appeared to exhibit preferential partitioning into condensates, which seems partially driven by the hydrophobic properties of their regions. The authors suggest that the chemical environment of the condensates may be favorable to lipid solvation and partitioning, however they do not rule out other mechanisms for localization of lipids to these environments, such as lipid binding proteins. The current findings also have implications for approaches to drug development as several well-known lipid modifying enzymes, such as PI3K, are disease associated and produce phospholipids found in these condensates. In total, the study provides evidence that phosphoinositides enter biomolecular condensates through phase separation providing a novel microenvironment for lipid signaling and metabolism.
Read the full article here:
Biomolecular condensates create phospholipid-enriched microenvironments
Nature Chemical Biology (2023)
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences