Molecular Weight: 716.75
Salt Form: N/A
Purity: >96%
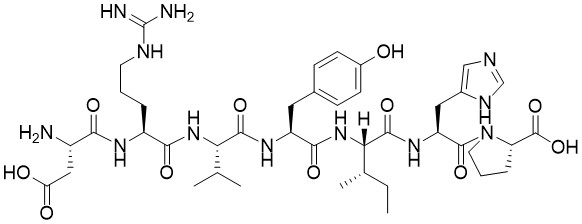
Sequence (3-letter): Ac-Val-Asp-Val-Ala-Asp-AMC
Sequence (1-letter): Ac-VDVAD-AMC
Storage: -20 °C or below
Ac-Val-Asp-Val-Ala-Asp-AMC is a fluorogenic substrate for Caspase 2. Caspase 2, part of the ICH-1 sub-family, is a cysteine protease with a poorly defined role in apoptosis. It associates with several apoptotic proteins such as death effector filament-forming Ced-4-like apoptosis protein (DEFCAP), RIP-associated Ich-1/Ced-3-homologue protein with a death domain (RAIDD), and apoptosis repressor with caspase recruitment domain (ARC). Caspase 2 can be activated by the PIDDosome formed by the association of PIDD1 and CRADD. Recent work demonstrates that Caspase-2 resides in the mitochondria and is essential for stress-induced apoptosis. Caspase-2 cleaves at an Asp residue with a preferred sequence of Val-Asp-Val-Ala-Asp-X.
Activity is quantified by release of free fluorescent 7-amino-4-methylcoumarin (AMC) which excites at 360-380 nm and emits at 440-460 nm.
References
1) Luca L. Fava, Florian J. Bock, Stephan Geley, Andreas Villunger “Caspase-2 at a Glance” Journal of Cell Science 2012 125: 5911-5915; doi: 10.1242/jcs.115105>
2) Lisa Bouchier-Hayes “The role of caspase-2 in stress-induced apoptosis” J Cell Mol Med. 2010 Jun; 14(6a): 1212–1224.
3) M Lopez-Cruzan, R Sharma, M Tiwari, S Karbach, D Holstein, C R Martin, J D Lechleiter & B Herman “Caspase-2 resides in the mitochondria and mediates apoptosis directly from the mitochondrial compartment” Cell Death Discovery vol 2, Article number: 16005 (2016).
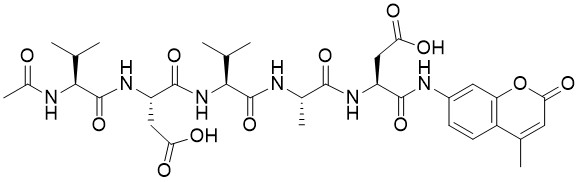
![Angiotensin I [Asn1,Val5], goosefish - Echelon Biosciences](https://www.echelon-inc.com/wp-content/uploads/2019/11/137-39.jpg)
![Sarilesin (Angiotensin II [Sar1,Ile8]), CAS 37827-06-8 - Echelon Biosciences](https://www.echelon-inc.com/wp-content/uploads/2019/11/131-63.jpg)