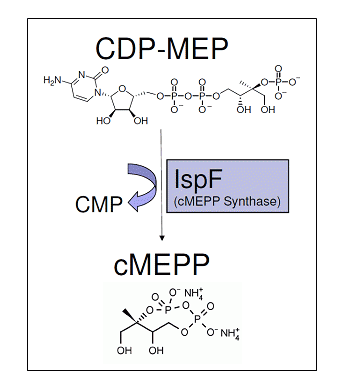
Recombinant 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (IspF) with an C-terminal His-tag, purified from E.coli using Ni-NTA column chromatography.
The MEP pathway is used by most bacteria, including all Gram-negative bacteria, for isoprenoid biosynthesis. Isoprenoids comprise one of the most diverse classes of compounds found in nature. With over 50,000 different isoprenoids identified to date, they exhibit a road range of structural complexity and are involved in a variety of biological functions [1]. Electron transport (quinones), stabilization of cell membranes (hopanoids and sterols), cell wall biosynthesis (dolichols), signal transduction (prenylated proteins), photosynthesis (cholorphylls) and modification of tRNAs are among the processes that involve isoprenoids [2]. Isopentyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are the precursors for all isoprenoid compounds and two unrelated essential pathways exist in nature for their biosynthesis. These two precursors are produced by either the mevalonate (MVA) or MEP pathway. The MVA pathway in found primarily is eukaryotes and archea, including humans, plant cytosol, and some Gram-positive bacteria, while the MEP pathway is utilized y most bacteria, including all Gram-negativs, and plant chloroplasts. Due to this natural distribution, the MEP pathway represents a promising target for development of novel antibacterial agents and herbicides [3].
Storage
Store product frozen below -20°C. Enzyme will be stable for at least 6 months at -20°C as undiluted stock. Freeze in working aliquots to avoid repeated thawing and freezing
References
1) Bochar, D.A.; Freisen, J.A.; Stauffacher, C.V. and Rodwell, V.W.(1999) in Comprehensive Natural Products Chemistry, (Cane, D. Ed.) Pergamon Press, Oxford, pp. 15-44.
2) Sacchettini, J.C. and Poulter, C.C. (1997) Science, 277(5333), 1788-9.
3) Testa, C.A.; Brown, M.J. (2003) Current Pharmceutical Biotechnology, 4, 248-259.