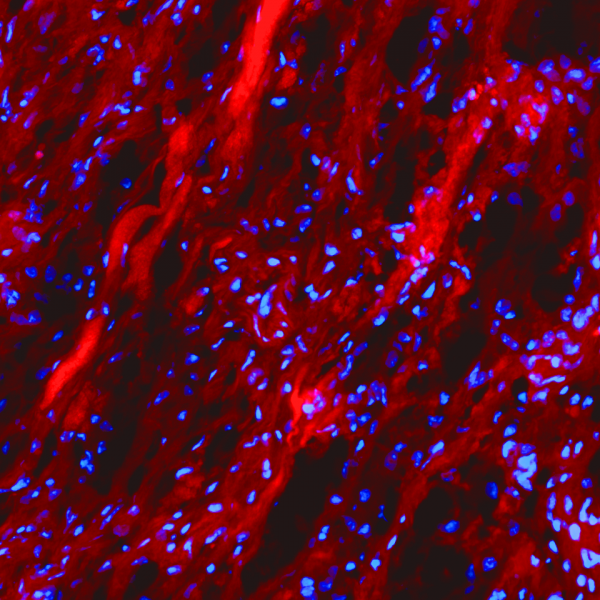
The Collagen Hybridizing Peptides are products of 3-Helix Inc.
The collagen hybridizing peptide (CHP) is a novel and unique peptide that specifically binds unfolded collagen chains, both in vitro and in vivo [1,2,3]. By sharing the Gly-X-Y repeating sequence of natural collagen, CHP has a strong capability to hybridize with denatured collagen chains by reforming the triple helical structure, in a fashion similar to DNA fragments annealing to complementary DNA strands. CHP is extremely specific: it has negligible affinity to intact collagen molecules due to lack of binding sites, and it is inert towards non-specific binding because of its neutral and hydrophilic nature.
CHP is a powerful histopathology tool which enables straightforward detection of inflammation and tissue damage caused by a large variety of diseases, as well as tissue remodeling during development and aging [3]. CHP robustly visualizes the pericellular matrix turnover caused by proteolytic migration of cancer cells within 3D collagen culture, without the use of synthetic fluorogenic matrices or genetically modified cells [4]. CHP can measure and localize mechanical injury to collagenous tissue at the molecular level [5]. It also enables assessment of collagen denaturation in decellularized extracellular matrix [6]. In addition, CHP can be used to specifically visualize collagen bands in SDS-PAGE gels without the need for western blot [7].
CHP come in three varieties: labeled with biotin (Catalog #: BIO) for avidin/streptavidin-mediated including HRP-enzyme methods, 5-FAM (Catalog #: FLU) for green fluorescence detection, and Cy3 (Catalog #: RED) for red fluorescence detection.
Applications: immunofluorescence, immunohistochemistry, SDS-PAGE (in-gel Western blot)
Shipping Conditions
Express shipping as powder at ambient temperature. Store at -20 °C upon arrival until use.
Storage
-20 °C as powder; 4 °C after reconstitution in water, no need to aliquot and freeze. Stable for at least 6 months in solution at 4 °C.
References
1) “Targeting and mimicking collagens via triple helical peptide assemblies” Curr Opin Chem Biol, 2013, 17, 968-975.
2) “Targeting collagen strand by photo-triggered triple-helix hybridization” Proceedings of the National Academy of Sciences of the United States of America, 2012, 109, 14767-14772. DOI: 10.1073/pnas.1209721109.
3) “In situ imaging of tissue remodeling with collagen hybridizing peptides.” ACS Nano, 2017, 11, 9825. DOI: 10.1021/acsnano.7b03150
4) “Visualizing collagen proteolysis by peptide hybridization: From 3D cell culture to in vivo imaging.” Biomaterials, 2018, DOI:10.1016/j.biomaterials.2018.08.039
5) “Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides.” Nat. Commun., 2017, 8, 14913, DOI: 10.1038/ncomms14913.
6) “Molecular assessment of collagen denaturation in decellularized tissues using the collagen hybridizing peptide.” Acta Biomater., 2017. 53, 268-278, DOI: 10.1016/j.actbio.2017.01.079
7) “Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides” Bioconjugate Chemistry, 2013, 24, 9-16. DOI: 10.1021/bc3005842.
8) “In tendons, differing physiological requirements lead to functionally distinct nanostructures.” Sci. Reports, 2018. DOI: DOI:10.1038/s41598-018-22741-8
Features
-More informative, reliable and convenient than zymography, DQ collagen, SHG, and TEM
-High affinity and unparalleled specificity to collagen with essentially no nonspecific binding
-Applicable to all types of collagen from all species, relying on collagen’s secondary structure instead of any defined sequence for binding
-A non-antibody approach with no species restrictions against any co-staining antibody
-Suitable for both frozen and paraffin-embedded sections with no need for antigen retrieval
-Small size (2% of IgG by MW) enabling facile tissue penetration and whole specimen staining without sectioning
-Stable in solution at 4 °C, eliminating the need to aliquot for storage