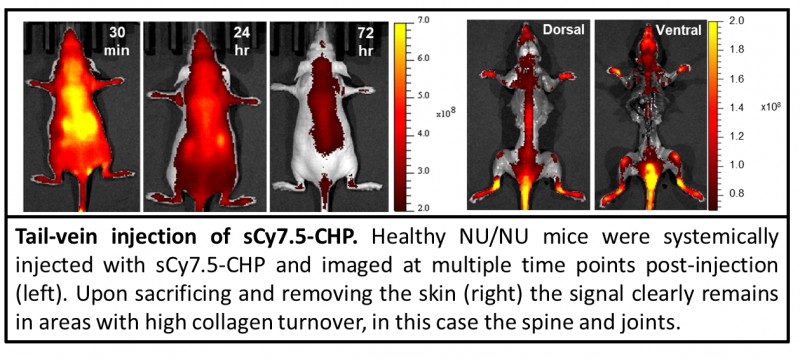
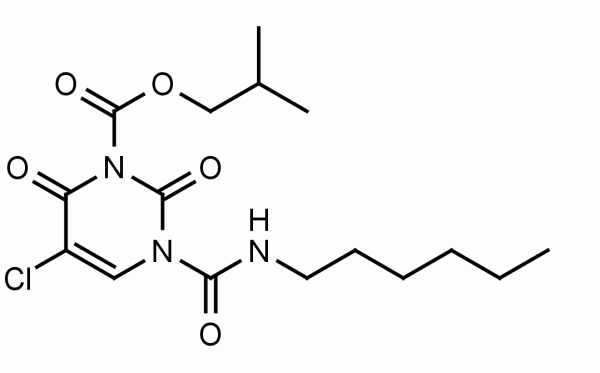
The collagen hybridizing peptide (CHP) is a novel and unique peptide that specifically binds unfolded collagen chains, both in vitro and in vivo.[1,2,3] By sharing the Gly-X-Y repeating sequence of natural collagen, CHP has a strong capability to hybridize with denatured collagen chains by reforming the triple helical structure, in a fashion similar to DNA fragments annealing to complementary DNA strands. CHP is extremely specific: it has negligible affinity to intact collagen molecules due to lack of binding sites, and it is inert towards non-specific binding because of its neutral and hydrophilic nature.
The in vivo CHP incorporates our newest sequence which allows them to be directly injected into animals without having to pre-activate them with a heating step. [4] Therefore, the in vivo CHPs will always be in their active form and will not self-hybridize once injected. [4] The sCy7.5-CHP is a powerful in vivo tool which enables straightforward detection of inflammation and tissue damage caused by a large variety of diseases, as well as tissue remodeling during development and aging.[3] CHP can measure and localize mechanical injury to collagenous tissue at the molecular level.[5]
The sCy7.5-CHPs are labeled with sulfonated-cyanine 7.5 dye for near-infrared fluorescence (NIRF) detection. This wavelength offers better tissue penetration while minimizing tissue autofluorescence.
Specificity: CHP binds to the unfolded triple-helical chains of all collagen types (e.g., I, II, III, IV, etc).[3,6]
Applications: in vivo imaging, small animal imaging
Features
- New sequence designed for in vivo use- does not need any pre-activation step unlike CHPs for histology
- Near-infrared imaging probe minimizes the chance of autofluorescence in the gut or certain tissues
- Can be used for systemic or in situ injection
- Signal lasts at target sites for days, allowing for imaging at multiple time points with a single dose
- High affinity and unparalleled specificity to collagen turnover with essentially no nonspecific binding
- Applicable to all types of collagen from all species, relying on collagen’s secondary structure instead of any defined sequence for binding
- Stable in solution under 4 °C up to three months, eliminating the need to aliquot for storage
Key Publications
1.Targeting and mimicking collagens via triple helical peptide assemblies. Curr. Opin. Chem. Biol., 2013. [link]
2.Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl. Acad. Sci. U.S.A., 2012. [link]
3.In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano, 2017. [link]
4.Visualizing collagen proteolysis by peptide hybridization: From 3D cell culture to in vivo imaging. Biomaterials, 2018. [link]
5.Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat. Commun., 2017. [link]
6.Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconjug. Chem., 2013. [link]
Additional Information
- sCy7.5-CHP dose can be adjusted based on animal model or application. Current dosing is based on a mouse model.