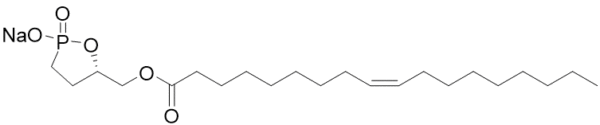
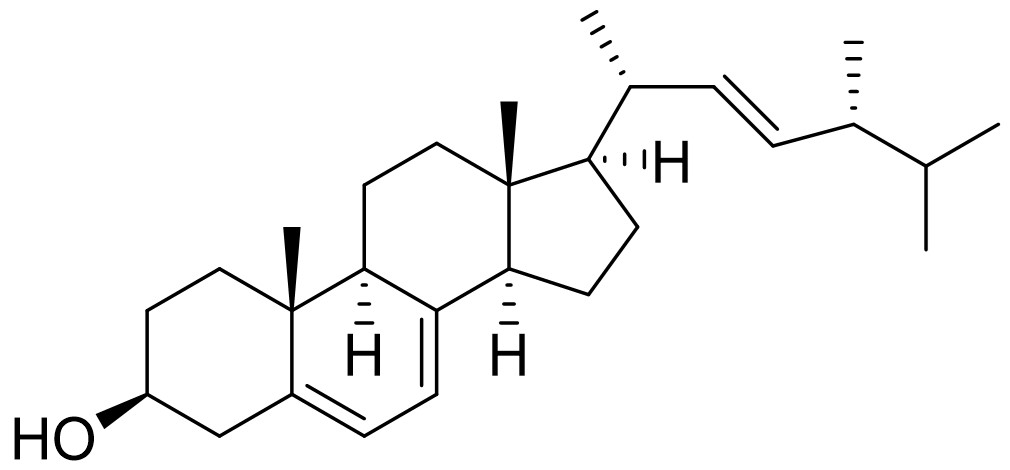
Oleoyl 3-carbacyclic Phosphatidic Acid (ccPA) is an analog of naturally occurring cyclic phosphatidic acid in which the sn-3 oxygen has been replaced with carbon making a stable phosphonate with is resistant to ring opening. ccPA is an analog of LPA but does not activate the LPA1-4 receptors. It also acts as a potent inhibitor of Autotaxin and can inhibit the metastasis of B16-F10 melanoma cells when injected into mice.
References
1) Baker, D. L., et al. “Carba Analogs of Cyclic Phosphatidic Acid Are Selective Inhibitors of Autotaxin and Cancer Cell Invasion and Metastasis” J. Biol. Chem. 2006, 281, 22786.
2) Uchiyama, A. et al. “Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid.” Biochim. Biophys. Acta 2007, 1771, 103.
3) Williams, J. R. et al “Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation” J. Biol. Chem. 2009, 284, 17304.