N-terminal His-tagged, recombinant human SHIP2 (truncated form containing SH2 domain and inositol 5-phophatase catalytic domain)
Storage: Store product > -70 ºC. Enzyme is stable for at least 6 months at -70 ºC as undiluted stock
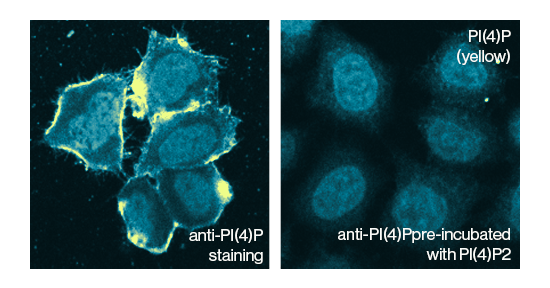
Specificity: SHIP2 selectively removes the phosphate from the 5’ position of the inositol ring of PtdIns(3,4,5)P3
Molecular Weight: 103 kDa
Specific Activity: request Certificate of Analysis (COA)
This His-tagged, recombinant human SHIP2 (truncated form containing SH2 domain and inositol 5-phophatase catalytic domain) is tested for activity against PIP3 and is purified from E.coli using Ni-NTA column chromatography.
SH2-containing 5`-inositol phosphatase 2 (SHIP2) is a lipid phosphatase which converts PI(3,4,5)P3 to PI(3,4)P2. This negative regulation of insulin signaling has a fundamental impact on insulin resistance. SHIP2 down-regulates insulin signaling and is present at higher levels in diabetes and obesity. Abnormalities in SHIP function are increasingly linked to disease, in particular in the role of SHIP as a negative regulator of cytokine and immune receptor signaling.
Publications
1. Drees, B. E., A. Weipert, et al. (2003). “Competitive fluorescence polarization assays for the detection of phosphoinositide kinase and phosphatase activity.” Comb Chem High Throughput Screen 6(4): 321-30.
2. Brooks, R., G. M. Fuhler, et al. (2010). “SHIP1 inhibition increases immunoregulatory capacity and triggers apoptosis of hematopoietic cancer cells.” J Immunol 184(7): 3582-9.
3. Fuhler, G. M. B., R, Toms, Iyer, Geno (2012). “Therapeutic potential of SHIP1 and SHIP2 inhibition in cancer cells.” Molecular Medicine 18: 65-75.
4. Agollah, G. D., et al. (2014). “Evidence for SH2 Domain-Containing 5′-Inositol Phosphatase-2 (SHIP2) Contributing to a Lymphatic Dysfunction.” PLoS ONE 9(11): e112548.
5. Proctor, A., et al. (2017). “Chemical fixation to arrest phospholipid signaling for chemical cytometry.” Journal of Chromatography A 1523(10): 97-106.
6. Willett, R., et al. (2017). “TFEB regulates lysosomal positioning by modulating TMEM55B expression and JIP4 recruitment to lysosomes.” Nature Communications 8(1): 1580.