Coauthored with Jeff Johnson and Melissa Burback
According to a May 2022 report from the World Health Organization (WHO), 3 million, or 5.3% of all deaths, every year worldwide result from harmful use of alcohol. A staggering 5.1% of the global burden of disease and injury is attributable to alcohol. Clearly, alcohol misuse is a significant global health concern.
Even though many people regularly consume alcohol, it is surprisingly difficult to accurately determine an individual’s level of alcohol consumption. Self-reported alcohol use is difficult to accurately determine due to the influence of external social factors and memory issues. Scientists and health care professionals have longed for objective biomarkers that would accurately report the timing and quantity of ingested alcohol. There are a number of candidate biomarkers, and the search for a sensitive and specific alcohol biomarker continues.
PEth as an alcohol biomarker
As a direct alcohol metabolite, phosphatidylethanol (PEth), is gaining acceptance as an accurate objective biomarker for measurement of past alcohol use. A cellular enzyme, Phospholipase D (PLD), converts phosphatidylcholine to phosphatidic acid in the presence of water. However, in the presence of ethanol, PLD generates a different modification to the lipid headgroup resulting in PEth. This modified lipid builds up in tissues as it is integrated into cellular membranes. There is no enzymatic degradation, so the lipid remains in red blood cells for weeks and up to more than a month after significant alcohol consumption.
Traditionally, Liquid Chromatography/Mass Spectrometry (LC/MS) has been used to measure PEth. This is a sensitive and powerful technique, but limitations include expensive equipment, specialized training, limited sample throughput, and lack of portability, and infrastructure needs (power, gases, etc.) for maintaining each system.
To address these limitations, Echelon has developed an immunoassay that measures PEth levels in a semi-quantifiable manner to supplement and pre-screen LC/MS analysis. Our assay recognizes multiple PEth species with sensitivity < 30 ng/ml and can reproducibly distinguish heavy and moderate alcohol consumption from light drinking and abstinence. It is also economical. Up to 80 samples can be analyzed per 96-well kit; costing < $10/sample.
PEth is quite stable when stored, extracted, and measured from dried blood spot (DBS) cards, allowing for flexibility in the collection process. Recent work at Echelon has found that blood collection via DBS, Tasso-M20® and Mitra®(VAMS®) further simplify the blood collection process and are compatible with the PEth ELISA. Our goal is to further simplify clinical laboratory processes, reduce time & cost, and expand the available options for clinical analysis of PEth.
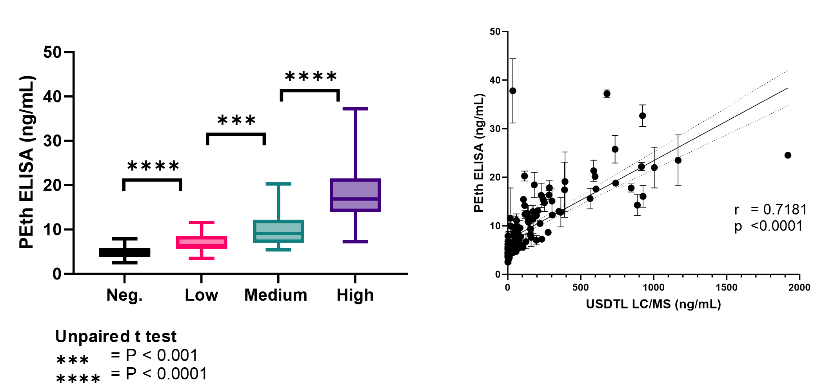
Figure 1: The Semi-Quantitative PEth ELISA demonstrates strong correlation between ELISA and MS PEth concentrations. (left) Quantitative averages of clinical samples binned within four groups based on PEth blood levels. (right) Correlation plot of values obtained in the PEth ELISA vs values obtained by mass spectrometry.
Echelon’s Semi-Quantitative PEth ELISA:
- Differentiates between four alcohol consumption categories in a quantitative fashion.
- Demonstrates significant correlation with reported LC/MS values.
- Is compatible with the Tasso-M20® and Mitra®(VAMS®) collection devices.
This 96-well immunoassay expands the availability of PEth analysis and is poised to become a valuable clinical tool in screening large numbers of samples in high-throughput workflows.
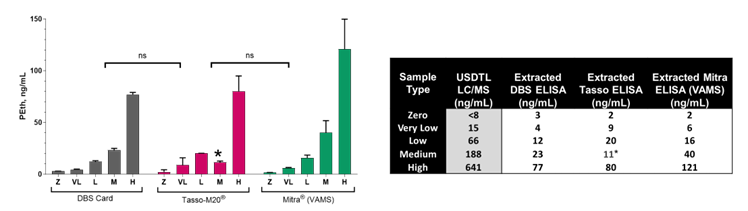
Figure 2: ELISA differentiates between five ex vivo generated PEth controls and displays no significant difference with blood collection devices. (left) Plots of various PEth blood levels obtained across three different blood collection devices. (right) Summary of data comparing blood collection devices paired with the PEth ELISA.
Additional data and details are found in a poster presented at the 2023 Research Society on Alcohol Meeting:
2023 RSA PosterReferences
- World Health Organization
- McKenna, H. et al, (2018) Evaluation of the psychometric properties of self-reported measures of alcohol consumption: a COSMIN systematic review. BioMedCentral, 13, Article number: 6
- Fakhari, S. et al, (2023) Old and New Biomarkers of Alcohol Abuse: Narrative Review. Journal of Clinical Medicine, 12, 2124
- Helander, A. et al, (2009) Molecular species of the alcohol biomarker phosphatidylethanol in human blood measured by LC-MS. Clin Chem 55(7):1395-405
- Agarwal, V. Fundamentals Of Liquid Chromatography Mass Spectrometry (LC-MS/MS).
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences