Since the discovery of hyaluronic acid (HA) in the 1930s, new functional roles of this special polymer continue to be discovered. The HA field is now substantial enough that a scientific society completely dedicated to HA research, the International Society for Hyaluronan Sciences (ISHAS), was founded in 2003 with ongoing biannual meetings. As the knowledge around HA biology has grown, there has been movement from basic structural and functional studies towards more clinical and therapeutic applications. While this is typical for any field, there is an existing need for new tools to study HA in vitro and in vivo. Here, we will discuss some research tools and methods in HA cell signaling and biomedical research.
HA Signaling and Size
HA is a key component of the extracellular matrix (ECM), a well characterized cellular scaffold found in most tissues. Beyond this simple support function, ECM proteins and polymers have key signaling functions. HA triggers cell signaling events by binding to cell surface receptors such as CD44, RHAMM, and LYVE-11. HA molecular weight (MW) also plays a significant role in cell signaling. For example, high and low MW HA trigger opposite effects in inflammation. It is believed that CD44 receptors are cross-linked by high MW HA and promotes the production of anti-inflammatory cytokines while low molecular weight HA (<200 kDa) trigger pro-inflammatory response through TLR2/TLR42. It was recently reported that a similar HA-CD44 mechanism enhances resistance to cellular stress, in which very high molecular weight HA (>6 MDa) prevents CD44 receptors from cross linking3. Current evidence suggests that the variance in signaling seen between high and low MW HA also has to do with clustering of different receptor types within a single HA molecule that lead to divergent signals.
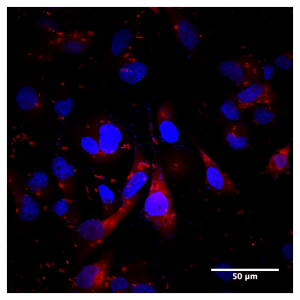
Figure 1: HA localization in high CD44 expressed MDA-MD-231 cell line using Texas Red HA
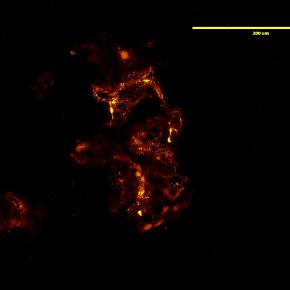
Figure 2: Detection of HA in HEK293 cells using a biotinylated HA-binding protein.
Localizing HA in the cell to receptor pools is key to studying HA signaling. One method to address this is to add fluorescent HA directly to cultured cells (Figure 1). Fluorescent HA is generated by directly conjugating a fluorophore to an HA molecule which can then be stably integrated to the endogenous ECM of the cells. Subsequent staining or labeling of specific receptors then allows for colocalization of HA polymers to distinct receptors. This is a powerful tool to study HA localization both in cell culture and in vivo and can be utilized for real time or live imaging. Direct localization of HA can also be achieved by utilizing HA binding proteins. HA has low immunogenicity, therefore it is difficult to generate antibodies for immunochemical staining. These binding proteins can be conjugated to enable visualization either directly or with a secondary marker (Figure 2). This allows for cell staining of HA in a manner similar to immunocytochemistry.
Synthesis and degradation of HA are also key steps during HA signaling to tune receptor binding and clustering. As mentioned above, the size of HA polymers greatly affects their interactions with specific receptors so the balance of HA synthase activity versus hyaluronidases is key. This facet of HA signaling can also be used to study HA function by modulating HA synthesis. Inhibitors for both HA synthase and hyaluronidase are available, and by using these inhibitors with the combination of other molecular biology techniques, it can provide researchers deeper insight into HA cell signaling mechanisms.
Molecular Weight Analysis and Purity
It is important to have well defined molecular weights and purity standards for any experiment where exogenous HA will be added to an experimental system. Many commercially available sources of HA are sourced from animals and have size ranges as wide as 8-2400 kDa, i.e. high polydispersity. Animal sources also carry the risk of high endotoxin levels which can promote inflammatory responses. Therefore, having discrete size HA with low endotoxin is crucial.
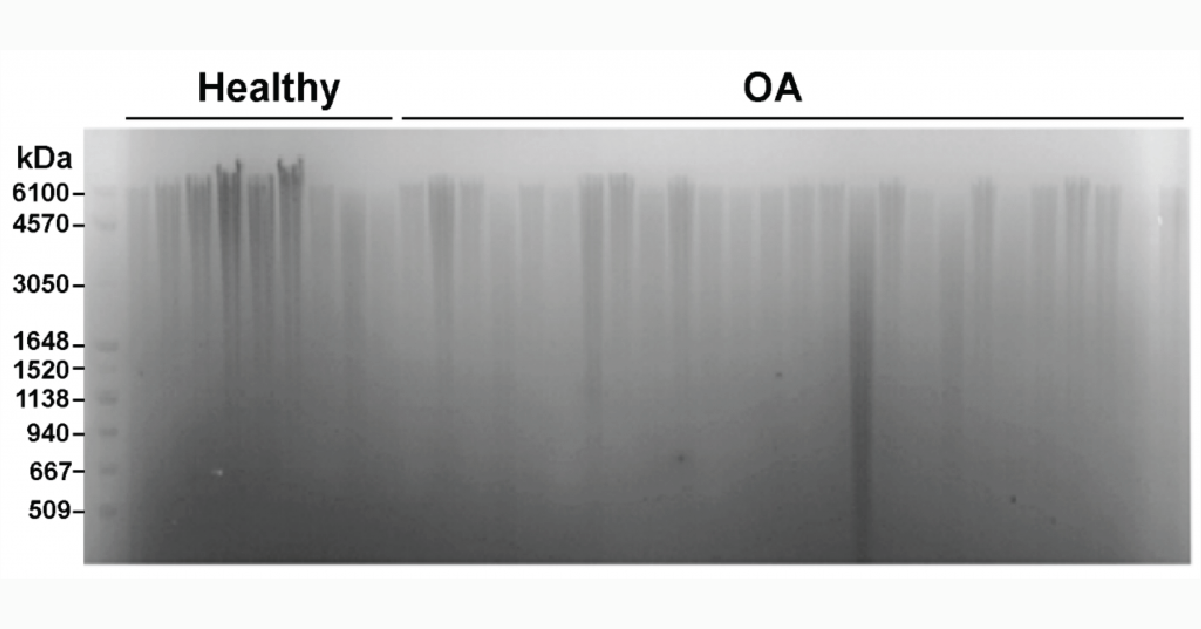
Precise analysis of HA polydispersity is commonly determined using gel permeation chromatography (GPC). However, this method is time consuming, has low throughput, and is fairly expensive. An alternative method that allows for multiple samples to be analyzed in parallel is gel electrophoresis (Figure 3). As shown below, several samples can be run at once and when paired with a molecular weight marker for HA the method can give a semi-quantitative readout of HA size and polydispersity3.
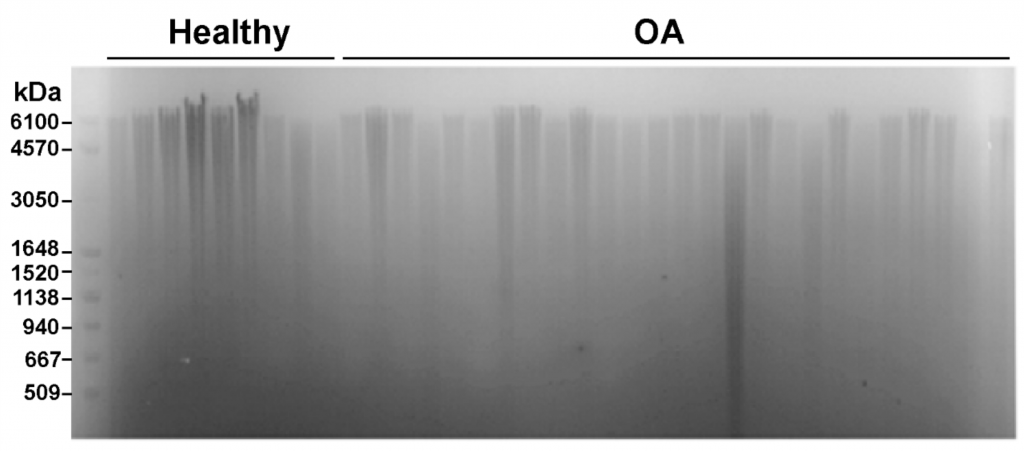
Figure 3: Measurement of HA distribution in healthy and osteoarthritis synovial fluid from equine samples using gel electrophoresis with Select-HA Hi Ladder (first lane)
Clinical Applications
HA is an excellent candidate for biomedical applications because it is naturally produced in the body and is a major component of the extracellular matrix. Injectable HA is already widely used to treat osteoarthritis. Many researchers are now studying HA-based scaffolds, wound healing, tissue engineering, and drug delivery applications5. This kind of research often involves chemical modification of HA to either extend the half-life or accelerate the degradation of HA applied to tissues. Measuring the amount of HA or the activity of relevant HA synthases or hyaluronidases can be used to monitor the efficiency of the modification. Moreover, using high-quality medical grade HA with lot-to-lot constancy as the base of these applications would not only save researchers’ time but also ensure smooth transition from bench to bedside.
Hyaluronic acid is a wonderful molecule. By using the right tools, we can explore the tremendous therapeutic applications of HA.
View All Hyaluronic Acid Tools
References
1. Vigetti, D. et al. (2014) Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta, 1840 (8), 2452-9.
2. Ruppert, S. M. et al. (2014) Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol Res 2014, 58 (2-3), 186-92.
3. Takasugi, M. et al. (2020) Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties. Nat Commun, 11 (1), 2376.
4. Fasanello, D. C. et al. (2021) Hyaluronic acid synthesis, degradation, and crosslinking in equine osteoarthritis: TNF-alpha-TSG-6-mediated HC-HA formation. Arthritis Res Ther, 23 (1), 218.
5. Dovedytis, M et al. (2020) Hyaluronic acid and its biomedical applications: A review. Engineered Regeneration, 1, 102-113.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences